Heap, I. The International Survey of Herbicide Resistant Weeds. Online. www.weedscience.com Accessed 1 March 2019. (2019).
Duke, S. O. & Powles, S. B. Glyphosate: a once-in-a-century herbicide. Pest Management Science. 64, 319–325 (2008).
Benbrook, C. M. Trends in glyphosate herbicide use in the United States and globally. Environmental Sciences. Europe. 28, 1–15 (2016).
Sammons, R. D. & Gaines, T. A. Glyphosate resistance: state of knowledge. Pest Management Science. 70, 1367–1377 (2014).
Heap, I., & Duke, S.O. Overview of glyphosate-resistant weeds worldwide. Pest Management Science. 74; https://doi.org/10.1002/ps.4760 (2017).
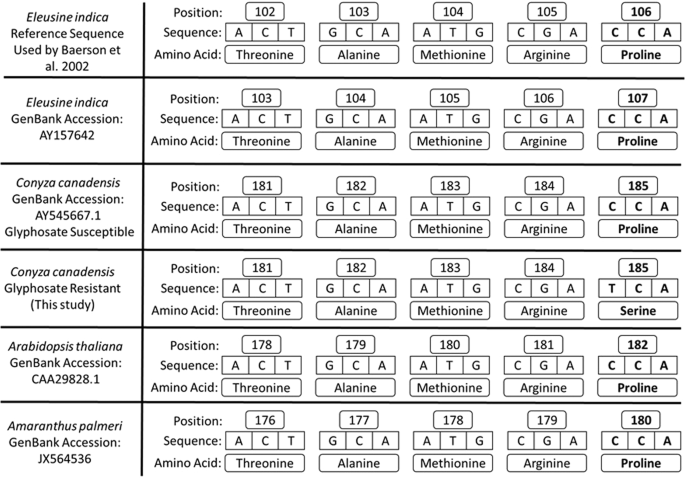
Baerson, S. R. et al. Glyphosate-resistant Goosegrass. Identification of a mutation in the target enzyme 5-enolpyruvylshikimate-3-phosphate synthase. Plant Physiology. 129, 1265–1275 (2002).
Alcántara-de la Cruz, R. et al. Target and non-target site mechanisms developed by glyphosate-resistant hairy beggarticks (Bidens pilosa L.) populations from Mexico. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2016.01492 (2016).
Yu, Q. et al. Evolution of a double amino acid substitution in the 5-enolpyruvylshikimate-3-phosphate synthase in Eleusine indica conferring high-level glyphosate resistance. Plant Physiology. 167, 1440–1447 (2015).
Han, H., Vila-Aiub, M. M., Jalaludin, A., Yu, Q. & Powles, S. B. A double EPSPS gene mutation endowing glyphosate resistance shows a remarkably high resistance cost. Plant, Cell, &. Environment. 40, 3031–3042 (2017).
Chen, J. et al. Mutations and amplification of EPSPS gene confer resistance to glyphosate in goosegrass (Eleusine indica). Planta. 242, 859–868 (2015).
Chen, J. et al. Characterization of Eleusine indica with gene mutation or amplication in EPSPS to glyphosate. Pesticide Biochemistry and Physiology. 143, 201–206 (2017).
Perotti, V. E. et al. A novel triple amino acid substitution in the EPSPS found in a high-level glyphosate-resistant Amaranthus hybridus population from Argentina. Pest Management Sciences. https://doi.org/10.1002/ps.5303 (2018).
VanGessel, M. J. Glyphosate-resistant horseweed from Delaware. Weed Science. 49, 703–705 (2001).
Zelaya, I. A., Owen, M. D. K. & VanGessel, M. J. Inheritance of evolved glyphosate resistance in horseweed (Conyza canadensis (I.) Cronq.). Theoretical Applied Genetics. 100, 58–70 (2004).
Zelaya, I. A., Owen, M. D. K. & VanGessel, M. J. Transfer of glyphosate resistance: evidence of hybridization in Conyza (Asteraceae). American Journal of Botany. 94, 660–673 (2007).
Feng, P. C. et al. Investigations into glyphosate-resistant horseweed (Conyza canadensis): retention, uptake, translocation, and metabolism. Weed Science. 52, 498–505 (2004).
Ge, X., d’Avignon, D. A., Ackerman, J. J. & Sammons, R. D. Rapid vacuolar sequestration: the horseweed glyphosate resistance mechanism. Pest Management Science. 66, 345–348 (2010).
Koger, C. H. & Reddy, K. N. Role of absorption and translocation in the mechanism of glyphosate resistance in horseweed (Conyza canadensis). Weed Science. 53, 84–89 (2005).
Dinelli, G. et al. Physiological and molecular insight on the mechanisms of resistance to glyphosate in Conyza canadensis (L.) Cronq. biotypes. Pesticide Biochemistry and Physiology. 86, 30–41 (2006).
Moretti, M. L. & Hanson, B. D. Reduced translocation is involved in resistance to glyphosate and paraquat in Conyza bonariensis and Conyza canadensis from California. Weed Research. 57, 25–34 (2017).
Hanson, B. D., Shrestha, A. & Shaner, D. L. Distribution of glyphosate-resistant horseweed (Conyza canadensis) and relationship to cropping systems in the Central Valley of California. Weed Science. 57, 48–53 (2009).
González-Torralva, F., Rojano-Delgado, A. M., Luque de Castro, M. D., Mülleder, N. & De Prado, R. Two non-target mechanisms are involved in glyphosate-resistant horseweed (Conyza canadensis L. Cronq.) biotypes. Journal of Plant Physiology. 169, 1673–1679 (2012).
Page, E. R. et al. Target and non-target site mechanisms confer resistance to glyphosate in Canadian accessions of Conyza canadensis. Weed Science. 66, 234–245 (2018).
Peng, Y. et al. Characterization of the horseweed (Conyza canadensis) transcriptome using GS-FLX 454 pyrosequencing and its application for candidate non-target herbicide resistance genes. Pest Management Science. 66, 1053–1062 (2010).
Peng, Y. et al. De novo genome assembly of the economically important weed horseweed using integrated data from multiple sequencing platforms. Plant Physiology. 166, 1241–1254 (2014).
Yuan, J. S. et al. Functional genomics analysis of horseweed (Conyza canadensis) with special reference to the evolution of non-target site glyphosate resistance. Weed Science. 58, 109–117 (2010).
d’Avignon, D. A. & Ge, X. In vivo NMR investigations of glyphosate influences on plant metabolism. Journal of Magnetic Resonance. 292, 59–72 (2018).
Tani, E., Chachalis, D. & Travlos, I. S. A glyphosate resistance mechanism in Conyza canadensis involves synchronization of EPSPS of ABC-transporter genes. Plant Molecular Biology Reporter. 33, 1721–1730 (2015).
Ge, X. et al. Glyphosate-resistant horseweed made sensitive to glyphosate: low-temperature suppression of glyphosate vacuolar sequestration revealed by 31PNMR. Pest Management Science. 67, 1215–1221 (2011).
Nol, N., Tsikou, D., Eid, M., Liverieratos, I. C. & Giannopolitis, C. N. Shikimate leaf disc assay for early detection of glyphosate resistance in Conyza canadensis and relative transcript levels of EPSPS and ABC transporter genes. Weed Research. 52, 233–241 (2012).
Mei, Y., Xu, Y., Wang, S., Qiu, L. & Zheng, M. Investigations of glyphosate resistance levels and target-site based resistance (TSR) mechanisms in Conyza canadensis (L.) from apple orchards around areas of Bohai seas and Loess Plateau in China. Pesticide Biochemistry and Physiology. 146, 7–12 (2018).
Beres, Z. T. et al. High levels of glyphosate resistance in Conyza canadensis from agricultural and non-agricultural sites in Ohio and Iowa. Scientific Reports. 8, 10483, https://doi.org/10.1038/s41598-018-28163-w (2018).
Nandula, V. K., Ray, J. D., Ribeiro, D. N., Pan, Z. & Reddy, K. N. Glyphosate resistance in tall waterhemp (Amaranthus tuberculatus) from Mississippi is due to both altered target-site and nontarget-site mechanisms. Weed Science. 61, 374–383 (2013).
Bostamam, Y., Malone, J. M., Dolman, F. C., Boutsalis, P. & Preston, C. Rigid ryegrass (Lolium rigidum) populations containing a target site mutation in EPSPS and reduced glyphosate translocation are more resistant to glyphosate. Weed Science. 60, 474–479 (2012).
Kaundun, S. S. et al. A novel P106L mutation in EPSPS and an unknown mechanism(s) act additively to confer resistance to glyphosate in a South African Lolium rigidum population. Journal Agricultural. Food Chemistry. 59, 3227–3233 (2011).
Dauer, J. T., Mortensen, D. A. & VanGessel, M. J. Temporal and spatial dynamics of long-distance Conyza canadensis seed dispersal. Journal of Applied Ecology. 44, 105–114 (2007).
Dauer, J. T., Luschei, E. C. & Mortensen, D. A. Effects of landscape composition on spread of an herbicide-resistant weed. Landscape Ecology. 24, 735–747 (2009).
Okada, M. et al. Evolution and spread of glyphosate resistance in Conyza canadensis in California. Evolutionary Applications. 6, 761–777 (2013).
González-Torralva, F., Gil-Humanes, J., Barro, F., Brants, I. & De Prado, R. Target site mutation and reduced translocation are present in a glyphosate-resistant Lolium multiflorum Lam. biotype from Spain. Plant Physiology and Biochemistry. 58, 16–22 (2012).
Weaver, S. E. The biology of Canadian weeds. Canadian Journal of Plant Science. 81, 867–875 (2001).
Regehr, D. L. & Bazzazz, F. A. The population dynamics of Erigeron canadensis, a successional winter annual. Journal of Ecology. 6, 923–933 (1979).
Tozzi, E., Lyons, E. M. & Van Acker, R. C. The effect of simulated winter warming spells on Canada fleabane [Conyza canadensis (L.) Cronq. var. canadensis] seeds and plants. Canadian Journal of Plant Science. 94, 963–969 (2014).
Shields, D. L., Dauer, J. T., VanGessel, M. J. & Neumann, G. Horseweed (Conyza canadensis) seed collected in the planetary boundary layer. Weed Science. 54, 1063–1067 (2006).
Davis, V. M., Kruger, G. R., Hallett, S. G., Tranel, P. J. & Johnson, W. G. Heritability of glyphosate resistance in Indiana horseweed (Conyza canadensis) populations. Weed Science. 58, 30–38 (2010).
Davis, V. M., Gibson, K. D., Bauman, T. T., Weller, S. C. & Johnson, W. G. Influence of weed management practices and crop rotation on glyphosate-resistant horseweed (Conyza canadensis) population dynamics and crop yield –years III and IV. Weed Science. 57, 417–426 (2009).
Dominguez-Valenzuela, J. A. et al. First confirmation and characterization of target and non-target site resistance to glyphosate in Palmer amaranth (Amaranthus palmeri) from Mexico. Plant Physiology and Biochemistry. 115, 212–218 (2017).
Bell, M. S., Hager, A. G. & Tranel, P. J. Multiple resistance to herbicides from four site-of-action groups in waterhemp (Amaranthus tuberculatus). Weed Science. 61, 460–468 (2013).
Murphy, B. P., Larran, A. S., Ackley, B., Loux, M. M. & Tranel, P. J. Survey of glyphosate-, atrazine- and lactofen-resistance mechanisms in Ohio waterhemp (Amaranthus tuberculatus) populations. Weed Science. 67, 296–302 (2019).
Ngo, T. D., Krishnan, M., Boutsalis, P., Gill, G. & Preston, C. Target-site mutations conferring resistance to glyphosate in feathertop Rhodes grass (Chloris virgata) populations in Australia. Pest Management Science. 74, 1094–1100 (2016).
de Carvalho, L. B. et al. Pool of resistance mechanisms to glyphosate in Digitaria insularis. Journal of Agricultural and Food Chemistry. 60, 615–622 (2012).
Alarcón-Reverte, R., Urzüa, J. & Fischer, A. J. Resistance to glyphosate in junglerice (Echinochloa colona) from California. Weed Science. 61, 48–54 (2012).
Alarcón-Reverte, R. et al. Concerted action of target-site mutations and high EPSPS activity in glyphosate-resistant junglerice (Echinochloa colona) from California. Pest Management Science. https://doi.org/10.1002/ps.3878 (2014).
Ng, C. H., Wickneswari, R., Salmijah, S., Teng, Y. T. & Ismail, B. S. Gene polymorphisms in glyphosate-resistant and –susceptible biotypes of Eleusine indica from Malaysia. Weed Research. 43, 108–115 (2003).
Ng, C. H., Wickneswari, R., Salmijah, S., Teng, Y. T. & Ismail, B. S. Glyphosate resistance in Eleusine indica (L.) Gaertn. from different origins and polymerase chain reaction amplification of specific alleles. Australian Journal of Agricultural Research. 55, 407–414 (2004).
Kaundun, S. S. et al. Importance of the P106S target-site mutation in conferring resistance to glyphosate in goosegrass (Eleusine indica) population from the Philippines. Weed Science. 56, 16–22 (2008).
Gherekhloo, J. et al. Pro-106-Ser mutation and EPSPS overexpression acting together simultaneously in glyphosate-resistant goosegrass (Eleusine indica). Scientific Reports. https://doi.org/10.1038/s41598-017-06772-1 (2017)
Takano, H. K. et al. Proline-106-EPSPS mutation imparting glyphosate resistance in goosegrass (Eleusine indica) emerges in South America. Weed Science. 67, 48–56 (2018).
Alcántara-de la Cruz, R. et al. First resistance mechanisms characterization in glyphosate-resistant Leptochloa virgata. Frontiers in Plant Science. 7, 1742, https://doi.org/10.3389/fpls.2016.01742 (2016).
Perez-Jones, A., Park, K. W., Polge, N., Colquhoun, J. & Mallory-Smith, C. A. Investigating the mechanisms of glyphosate resistance in Lolium multiflorum. Planta. 226, 395–404 (2007).
Jasieniuk, M. et al. Glyphosate-resistant Italian ryegrass (Lolium multiflorum) in California: distribution, response to glyphosate, and molecular evidence for an altered target enzyme. Weed Science. 56, 496–502 (2008).
Wakelin, A. M. & Preston, C. A target-site mutation is present in a glyphosate-resistant Lolium rigidum population. Weed Research. 46, 432–440 (2006).
Yu, Q., Cairns, A. & Powles, S. Glyphosate, paraquat and ACCase multiple herbicide resistance evolved in a Lolium rigidum biotype. Planta. 225, 499–513 (2007).
Simarmata, M. & Penner, D. The basis for glyphosate resistance in rigid ryegrass (Lolium rigidum) population. Weed Research. 56, 181–188 (2008).
Collavo, A. & Sattin, M. Resistance to glyphosate in Lolium rigidum selected in Italian perennial crops: bioevaluation, management and molecular bases of target-site resistance. Weed Research. 52, 16–24 (2012).
Cross, R. B. et al. A Pro-106 to Ala substitution is associated with resistance to glyphosate in annual bluegrass (Poa annua). Weed Science. 63, 613–622 (2015).
Source: Ecology - nature.com


