Gurr, G. M., Wratten, S. D., Landis, D. A. & You, M. S. Habitat management to suppress pest populations: progress and prospects. Annu. Rev. Entomol. 62, 91–109 (2017).
Puech, C., Poggi, S., Baudry, J. & Aviron, S. Do farming practices affect natural enemies at the landscape scale? Landscape Ecol. 30, 125–140 (2015).
Ramsden, M. W., Menéndez, R., Leather, S. R. & Wäckers, F. Optimizing field margins for biocontrol services: The relative role of aphid abundance, annual floral resources, and overwinter habitat in enhancing aphid natural enemies. Agr. Ecosyst. Environ. 199, 94–104 (2015).
Skellern, M. P. & Cook, S. M. The potential of crop management practices to reduce pollen beetle damage in oilseed rape. Arthropod-Plant Inte. 12, 867–879 (2017).
Heimoana, V. et al. Integrating spatially explicit molecular and ecological methods to explore the significance of non-crop vegetation to predators of brassica pests. Agr. Ecosyst. Environ. 239, 12–19 (2017).
Berndt, L. A. & Wratten, S. D. Effects of alyssum flowers on the longevity, fecundity, and sex ratio of the leafroller parasitoid Dolichogenidea tasmanica. Biol.Control 32, 65–69 (2005).
Pandey, S., Rahman, A. & Gurr, G. M. Australian native flowering plants enhance the longevity of three parasitoids of brassica pests. Entomol. Exp. Appl. 166, 265–276 (2018).
Araj, S. E., Wratten, S., Lister, A., Buckley, H. & Ghabeish, I. Searching behavior of an aphid parasitoid and its hyperparasitoid with and without floral nectar. Biol. Control 57, 79–84 (2011).
Zhu, P. Y. et al. Selective enhancement of parasitoids of rice Lepidoptera pests by sesame (Sesamum indicum) flowers. Biocontrol 60, 157–167 (2015).
Delisle, J. F., Shipp, L. & Brodeur, J. Apple pollen as a supplemental food source for the control of western flower thrips by two predatory mites, Amblyseius swirskii and Neoseiulus cucumeris (Acari: Phytoseiidae), on potted chrysanthemum. Exp. Appl. Acarol. 65, 495–509 (2015).
van Rijn, P. C. J., Kooijman, J. & Wäckers, F. L. The contribution of floral resources and honeydew to the performance of predatory hoverflies (Diptera: Syrphidae). Biol. Control 67, 32–38 (2013).
Villa, M., Santos, S. A. P., Mexia, A., Bento, A. & Pereira, J. A. Wild flower resources and insect honeydew are potential food items for Elasmus flabellatus. 37, 15, https://doi.org/10.1007/s13593-017-0423-0 (2017).
Fonseca, M. M., Lima, E., Lemos, F., Venzon, M. & Janssen, A. Non-crop plant to attract and conserve an aphid predator (Coleoptera: Coccinellidae) in tomato. Biol.Control 115, 129–134 (2017).
Nelson, J. L. et al. Arthropod communities in warm and cool grass riparian buffers and their influence on natural enemies in adjacent crops. Agr. Ecosyst. Environ. 257, 81–91 (2018).
Lu, Z. X. et al. Mechanisms for flowering plants to benefit arthropod natural enemies of insect pests: Prospects for enhanced use in agriculture. Insect Sci. 21, 1–12 (2014).
Gillespie, M. A. K., Gurr, G. M. & Wratten, S. D. Beyond nectar provision: the other resource requirements of parasitoid biological control agents. Entomol. Exp. Appl. 159, 207–221 (2016).
Arnó, J., Oveja, M. F. & Gabarra, R. Selection of flowering plants to enhance the biological control of Tuta absoluta using parasitoids. Biol. Control 122, 41–50 (2018).
Lee, J. C. & Heimpel, G. E. Floral resources impact longevity and oviposition rate of a parasitoid in the field. J. Anim. Ecol. 77, 565–572 (2008).
Wäckers, F. L., Romeis, J. & van Rijn, P. Nectar and pollen feeding by insect herbivores and implications for multitrophic interactions. Annu. Rev. Entomol. 52, 301–323 (2007).
Baggen, L. R., Gurr, G. M. & Meats, A. Flowers in tri-trophic systems: mechanisms allowing selective exploitation by insect natural enemies for conservation biological control. In Proceedings of the 10th International Symposium on Insect-Plant Relationships (eds Simpson, S. J., Mordue, A. J., & Hardie, J.) 155–161 (Springer Netherlands, 1999).
Winkler, K., Wäckers, F. L., Kaufman, L. V., Larraz, V. & van Lenteren, J. C. Nectar exploitation by herbivores and their parasitoids is a function of flower species and relative humidity. Biol. Control 50, 299–306 (2009).
Winkler, K., Wäckers, F. L., Termorshuizen, A. J. & van Lenteren, J. C. Assessing risks and benefits of floral supplements in conservation biological control. Biocontrol 55, 719–727 (2010).
Begum, M., Gurr, G. M., Wratten, S. D. & Nicol, H. I. Flower color affects tri-trophic-level biocontrol interactions. Biol.Control 30, 584–590 (2004).
Belz, E., Kölliker, M. & Balmer, O. Olfactory attractiveness of flowering plants to the parasitoid Microplitis mediator: potential implications for biological control. Biocontrol 58, 163–173 (2013).
Wäckers, F. L. Assessing the suitability of flowering herbs as parasitoid food sources: flower attractiveness and nectar accessibility. Biol. Control 29, 307–314 (2004).
Jervis, M. Functional and evolutionary aspects of mouthpart structure in parasitoid wasps. Biol. J. Lin. Soc. 63, 461–493 (1998).
Patt, J. M., Hamilton, G. C. & Lashomb, J. H. Foraging success of parasitoid wasps on flowers: interplay of insect morphology, floral architecture and searching behavior. Entomol. Exp. Appl. 83, 21–30 (1997).
Bianchi, F. J. J. A. & Wäckers, F. L. Effects of flower attractiveness and nectar availability in field margins on biological control by parasitoids. Biol.Control 46, 400–408 (2008).
Kugimiya, S., Uefune, M., Shimoda, T. & Takabayashi, J. Orientation of the parasitic wasp, Cotesia vestalis (Haliday) (Hymenoptera: Braconidae), to visual and olfactory cues of field mustard flowers, Brassica rapa L. (Brassicaceae), to exploit food sources. Appl. Entomol. Zool. 45, 369–375 (2010).
Raguso, R. A. Why are some floral nectars scented? Ecol. 85, 1486–1494 (2004).
Raguso, R. A. Floral scent, olfaction, and scent-driven foraging behavior. In Cognitive Ecology of Pollination: Animal Behavior and Floral Evolution (eds Chittka, L. & Thomson, J. D.) 83–105 (Cambridge University Press, 2001).
Foti, M. C. et al. Chemical ecology meets conservation biological control: identifying plant volatiles as predictors of floral resource suitability for an egg parasitoid of stink bugs. J. Pest Sci. 90, 299–310 (2017).
Kessler, D. & Baldwin, I. T. Making sense of nectar scents: the effects of nectar secondary metabolites on floral visitors of Nicotiana attenuata. Plant J. 49, 840–854 (2006).
Araj, S. E. & Wratten, S. D. Comparing existing weeds and commonly used insectary plants as floral resources for a parasitoid. Biol. Control 81, 15–20 (2015).
Zhu, P. Y. et al. Laboratory screening supports the selection of sesame (Sesamum indicum) to enhance Anagrus spp. parasitoids (Hymenoptera: Mymaridae) of rice planthoppers. Biol. Control 64, 83–89 (2013).
Witting-Bissinger, B. E., Orr, D. B. & Linker, H. M. Effects of floral resources on fitness of the parasitoids Trichogramma exiguum (Hymenoptera: Trichogrammatidae) and Cotesia congregata (Hymenoptera: Braconidae). Biol. Control 47, 180–186 (2008).
Furlong, M. J., Wright, D. J. & Dosdall, L. M. Diamondback moth ecology and management: problems, progress, and prospects. Annu. Rev. Entomol. 58, 517–541 (2013).
Zalucki, M. P. et al. Estimating the economic cost of one of the world’s major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): just how long is a piece of string? J. Econ. Entomol. 105, 1115–1129 (2012).
Talekar, N. S. & Shelton, A. M. Biology, ecology, and management of the diamondback moth. Annu. Rev. Entomol. 38, 275–301 (1993).
Whalon, M. E., Mota-Sanchez, D. & Hollingworth, R. M. Arthropod pesticide resistance database, http://www.pesticideresistance.org/index.php (2016).
APRD. Arthropod pesticide resistance database, http://www.pesticideresistance.org (2015).
Rezaei, M., Karimzadeh, J., Shakarami, J. & Jafary, S. Side effects of insecticides on the adult longivity of Cotesia vestalis, a larval parasitoid of the diamondback moth, Plutella xylostella. J. Entomol. Zool. Studies 2, 49–51 (2014).
Liu, S. S., Wang, X. G., Guo, S. J., He, J. H. & Shi, Z. H. Seasonal abundance of the parasitoid complex associated with the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) in Hangzhou, China. Bull. Entomol. Res. 90, 221–231 (2000).
Ayalew, G., Löhr, B., Baumgärtner, J. & Ogol, C. K. P. O. Diamondback moth (Plutella xylostella L.) (Lepidoptera: Plutellidae) and its parasitoids in Ethiopia. In Improving biocontrol of Plutella xylostella Proceedings of the International Symposium (eds Kirk, A. A. & Bordat, D.) 140–143 (2004).
Talekar, N. S. & Yang, J. C. Characteristic of parasitism of diamondback moth by two larval parasites. Entomophaga 36, 95–104 (1991).
Sarfraz, M., Keddie, A. B. & Dosdall, L. M. Biological control of the diamondback moth, Plutella xylostella: A review. Biocontrol Sci. Techn. 15, 763–789 (2005).
Kugimiya, S., Shimoda, T., Tabata, J. & Takabayashi, J. Present or past herbivory: A screening of volatiles released from Brassica rapa under caterpillar attacks as attractants for the solitary parasitoid, Cotesia vestalis. J. Chem. Ecol. 36, 620–628 (2010).
Soyelu, O. J. Effect of nutrition on Catesia Plutellae (Hymenoptera: Braconidae) and its parasitism on the diamondback moth, Plutella Xylostella (Lepidoptera: Plutellidae) Doctor thesis, University of Fort Hare (2010).
Jervis, M. A., Heimpel, G. E., Ferns, P. N., Harvey, J. A. & Kidd, N. A. C. Life-history strategies in parasitoid wasps: a comparative analysis of ‘ovigeny’. J. Anim. Ecol. 70, 442–458 (2001).
Zhang, Y. B., Liu, W. X., Wang, W., Wan, F. H. & Li, Q. Lifetime gains and patterns of accumulation and mobilization of nutrients in females of the synovigenic parasitoid, Diglyphus isaea Walker (Hymenoptera: Eulophidae), as a function of diet. J. Insect Physiol. 57, 1045–1052 (2011).
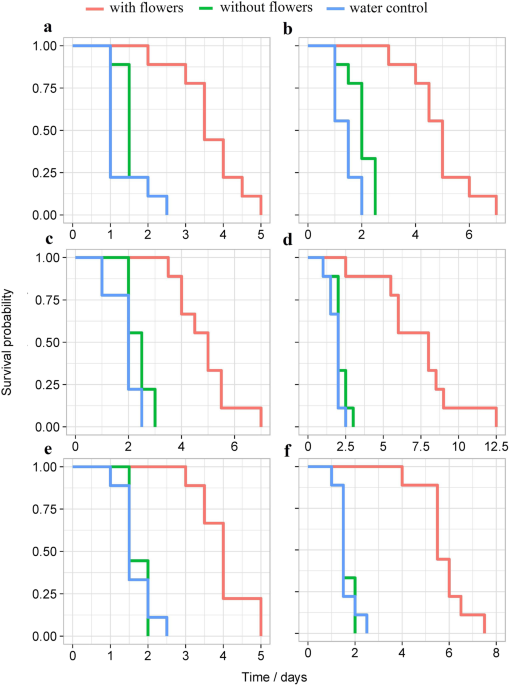
Mitsunaga, T., Shimoda, T. & Yano, E. Influence of food supply on longevity and parasitization ability of a larval endoparasitoid, Cotesia plutellae (Hymenoptera: Braconidae). Appl. Entomol. Zool. 39, 691–697 (2004).
Shimoda, T. et al. A food-supply device for maintaining Cotesia vestalis, a larval parasitoid of the diamondback moth Plutella xylostella, in greenhouses. Biocontrol 59, 681–688 (2014).
Ghosh, A, & Manchanda, N. Phytoremediation of Heavy Metals from Water of Yamuna River by Tagetes patula, Bassica scoparia, Portulaca grandiflora. Asian Plant Researchm, 1–14 (2019).
Lavandero, B., Wratten, S. D., Didham, R. K. & Gurr, G. M. Increasing floral diversity for selective enhancement of biological control agents: A double-edged sward? Basic Appl. Ecol. 7, 236–243 (2006).
Aparicio, Y., Gabarra, R. & Arno, J. Attraction of Aphidius ervi (Hymenoptera: Braconidae) and Aphidoletes aphidimyza (Diptera: Cecidomyiidae) to sweet alyssum and assessment of plant resources effects on their fitness. J. Econ. Entomol. 111, 533–541 (2018).
Lee, J. C., Heimpel, G. E. & Leibee, G. L. Comparing floral nectar and aphid honeydew diets on the longevity and nutrient levels of a parasitoid wasp. Entomol. Exp. Appl. 111, 189–199 (2004).
Nafziger, T. D. & Fadamiro, H. Y. Suitability of some farmscaping plants as nectar sources for the parasitoid wasp, Microplitis croceipes (Hymenoptera: Braconidae): Effects on longevity and body nutrients. Biol. Control 56, 225–229 (2011).
Borghi, M. & Fernie, A. R. Floral metabolism of sugars and amino acids: implications for pollinators’ preferences and seed and fruit set. Plant Physiol. 175, 1510–1524 (2017).
Baker, H. G. Non-sugar chemical constituents of nectar. Apidologie, 349–356 (1977).
Petanidou, T., Van Laere, A., Ellis, W. N. & Smets, E. What shapes amino acid and sugar composition in Mediterranean floral nectars? Oikos 115, 155–169 (2006).
Giron, D., Pincebourde, S. & Casas, J. Lifetime gains of host-feeding in a synovigenic parasitic wasp. Physiol. Entomol. 29, 436–442 (2004).
Casas, J. et al. Lifetime nutrient dynamics reveal simultaneous capital and income breeding in a parasitoid. Ecol. 86, 545–554 (2005).
Lee, J. C. & Heimpel, G. E. Impact of flowering buckwheat on Lepidopteran cabbage pests and their parasitoids at two spatial scales. Biol. Control 34, 290–301 (2005).
Shrestha, B., Finke, D. L. & Piñero, J. C. The “Botanical Triad’: The presence of insectary plants enhances natural enemy abundance on trap crop plants in an organic cabbage agro-ecosystem. Insects 10, 181 (2019).
Géneau, C. E., Wäckers, F. L., Luka, H., Daniel, C. & Balmer, O. Selective flowers to enhance biological control of cabbage pests by parasitoids. Basic Appl. Ecol. 13, 85–93 (2012).
Mathur, V. et al. An ecogenomic analysis of herbivore-induced plant volatiles in Brassica juncea. Mol. Ecol. 22, 6179–6196 (2013).
Uefune, M. et al. Application of synthetic herbivore-induced plant volatiles causes increased parasitism of herbivores in the field. J. Appl. Entomol. 136, 561–567 (2012).
Winkler, K., Wäckers, F. L., Stingli, A. & van Lenteren, J. C. Plutella xylostella (diamondback moth) and its parasitoid Diadegma semiclausum show different gustatory and longevity responses to a range of nectar and honeydew sugars. Entomol. Exp. Appl. 115, 187–192 (2005).
Winkler, K., Wäckers, F. L., Buitriago, L. & van Lenteren, J. C. Herbivores and their parasitoids show differences in abundance on eight different nectar producing plants. In Proceedings of the Netherlands Entomological Society Meeting Vol. 16, 125–130 (Wageningen University, Netherlands, 2005).
De-Groot, M., Winkler, K. & Potting, R. P. J. Testing the potential of White mustard (Sinapis alba) and Sweet alyssum (Lobularia maritima) as trap crops for the Diamondback moth Plutella xylostella. Proc. Neth. Entomol. Soc. Meet. 16, 117–123 (2005).
Khan, Z. R., James, D. G., Midega, C. A. O. & Pickett, J. A. Chemical ecology and conservation biological control. Biol. Control 45, 210–224 (2008).
Jonsson, M., Wratten, S. D., Landis, D. A. & Gurr, G. M. Recent advances in conservation biological control of arthropods by arthropods. Biol. Control 45, 172–175 (2008).
Simpson, M. et al. Attract and reward: combining chemical ecology and habitat manipulation to enhance biological control in field crops. J. Appl. Ecol. 48, 580–590 (2011).
Orre-Gordon, G. U. S., Wratten, S. D., Jonsson, M., Simpson, M. & Hale, R. ‘Attract and reward’: Combining a herbivore-induced plant volatile with floral resource supplementation – Multi-trophic level effects. Biol. Control 64, 106–115 (2013).
Sisterson, M. S. & Averill, A. L. Costs and benefits of food foraging for a Braconid parasitoid. J. Insect Behav. 15, 571–588 (2002).
Jamont, M., Dubois-Pot, C. & Jaloux, B. Nectar provisioning close to host patches increases parasitoid recruitment, retention and host parasitism. Basic Appl. Ecol. 15, 151–160 (2014).
Ribeiro, A. L. & Gontijo, L. M. Alyssum flowers promote biological control of collard pests. Biocontrol 62, 185–196 (2017).
Badenes-Pérez, R. F. Trap Crops and Insectary Plants in the Order Brassicales. Ann. Entomol. Soc. Am. 112, 318–329 (2018).
Source: Ecology - nature.com


