UN. The Sustainable Development Goals Report. (United Nations, New York, 2018).
Robinson, T. P. & Pozzi, F. Mapping Supply and Demand for Animal-Source Foods to 2030, Animal Production Health Working Paper (Food Agric Org, Rome N° 164, 2011).
Suweis, S., Carr, J. A., Maritan, A., Rinaldo, A. & D’Odorico, P. Resilience and reactivity of global food security. PNAS 112, 6902–6907 (2015).
FAO. The Future of Food and Agriculture – Trends and Challenges. (Rome, 2017).
Herrero, M. et al. Livestock and the environment: what have we learned in the past decade? Annu. Rev. Environ. Resour. 40, 177–202 (2015).
Van Boeckel, T. P. et al. Global trends in antimicrobial use in food animals. PNAS 112, 5649–5654 (2015).
Schar, D., Sommanustweechai, A., Laxminarayan, R. & Tangcharoensathien, V. Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: optimizing use and addressing antimicrobial resistance. PLoS Med. 15, e1002521 (2018).
Springmann, M., Godfray, H. C. J., Rayner, M. & Scarborough, P. Analysis and valuation of the health and climate change cobenefits of dietary change. Proc. Natl Acad. Sci. USA 113, 4146–4151 (2016).
Thilsted, S. H. et al. Sustaining healthy diets: the role of capture fisheries and aquaculture for improving nutrition in the post-2015 era. Food Policy 61, 126–131 (2016).
Froehlich, H. E., Runge, C. A., Gentry, R. R., Gaines, S. D. & Halpern, B. S. Comparative terrestrial feed and land use of an aquaculture-dominant world. Proc. Natl Acad. Sci. USA 115, 5295–5300 (2018).
World Bank. Fish to 2030: Prospects for Fisheries and Aquaculture (English). Agriculture and Environmental Services Discussion Paper; No. 3 (World Bank Group, Washington DC, 2013).
FAO. The State of World Fisheries and Aquaculture 2018 – Meeting the Sustainable Development Goals (Rome, 2018).
Béné, C. et al. Contribution of fisheries and aquaculture to food security and poverty reduction: assessing the current evidence. World Dev. 79, 177–196 (2016).
Belton, B., Bush, S. R. & Little, D. C. Not just for the wealthy: rethinking farmed fish consumption in the Global South. Glob. Food Sec. 16, 85–92 (2018).
Marcos-López, M., Gale, P., Oidtmann, B. C. & Peeler, E. J. Assessing the impact of climate change on disease emergence in freshwater fish in the United Kingdom. Transbound. Emerg. Dis. 57, 293–304 (2010).
Karvonen, A., Rintamäki, P., Jokela, J. & Valtonen, E. T. Increasing water temperature and disease risks in aquatic systems: climate change increases the risk of some, but not all, diseases. Int. J. Parasitol. 40, 1483–1488 (2010).
Vezzulli, L. et al. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc. Natl Acad. Sci. USA 113, E5062–E5071 (2015).
Bondad-Reantaso & Melba. Acute hepatopancreatic necrosis disease (AHPND) of penaeid shrimps: Global perspective. SEAFDEC http://hdl.handle.net/10862/3084 (2016)
FAO. Impacts on Climate Change on Fisheries and Aquaculture – Synthesis on Current Knowledge, Adaptation and Mitigation Options (Rome, 2018).
Mohanty, B. R. & Sahoo, P. K. Edwardsiellosis in fish: a brief review. J. Biosci. 32, 1331–1344 (2007).
Kayansamruaj, P., Pirarat, N., Hirono, I. & Rodkhum, C. Increasing of temperature induces pathogenicity of Streptococcus agalactiae and the up-regulation of inflammatory related genes in infected Nile tilapia (Oreochromis niloticus). Vet. Microbiol. 172, 265–271 (2014).
Bontad-Reantaso, M. G. Acute hepatopancreatic necrosis disease (AHND) of penaeid shrimps: Global perspective. Global perspective. In R. V. Pakingking Jr, E. G. T. de Jesus-Ayson, & B. O. Acosta (Eds.), Addressing Acute Hepatopancreatic Necrosis Disease (AHPND) and Other Transboundary Diseases for Improved Aquatic Animal Health in Southeast Asia: Proceedings of the ASEAN Regional Technical Consultation on EMS/AHPND and Other Transboundary Disease for Improved Aquatic Animal Health in Southeast Asia, 22-24 February 2016, Makati City, Philippines (pp. 16-23) (Aquaculture Department, Southeast Asian Fisheries Development Center, Tigbauan, Iloilo, Philippines, 2016).
Cabello, F. C., Godfrey, H. P., Buschmann, A. H. & Dölz, H. J. Aquaculture as yet another environmental gateway to the development and globalisation of antimicrobial resistance. Lancet Infect. Dis. 16, e127–e133 (2016).
CDC. Antibiotic Resistance Threats in the United States, 2019 (U.S. Department of Health and Human Services, CDC, Atlanta, GA, 2019).
Cassini, A. et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect. Dis. 19, 56–66 (2019).
Su, H. et al. Occurrence and temporal variation of antibiotic resistance genes (ARGs) in shrimp aquaculture: ARGs dissemination from farming source to reared organisms. Sci. Total Environ. 607–608, 357–366 (2017).
Jang, H. M. et al. Prevalence of antibiotic resistance genes from effluent of coastal aquaculture, South Korea. Environ. Pollut. 233, 1049–1057 (2018).
Miranda, C. D., Godoy, F. A. & Lee, M. R. Current status of the use of antibiotics and the antimicrobial resistance in the Chilean salmon farms. Front Microbiol 9, 1284 (2018).
Marti, E., Variatza, E. & Balcazar, J. L. The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol. 22, 36–41 (2014).
Seiler, C. & Berendonk, T. U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol 3, 399 (2012).
Pal, C., Bengtsson-Palme, J., Kristiansson, E. & Larsson, D. G. J. Co-occurrence of resistance genes to antibiotics, biocides and metals reveals novel insights into their co-selection potential. BMC Genomics 16, 964 (2015).
Lin, C. K. Prawn culture in Taiwan: what went wrong. World Aquacult. Soc. 20, 19–20 (1989).
Yang, J. H. et al. Antibiotic-induced changes to the host metabolic environment inhibit drug efficacy and alter immune function. Cell Host Microbe 22, 757–765 (2017).
Azzam, M. I., Ezzat, S. M., Othman, B. A. & El-Dougdoug, K. A. Antibiotics resistance phenomenon and virulence ability in bacteria from water environment. Water Sci. 31, 109–121 (2017).
MacFadden, D. R., McGough, S. F., Fisman, D., Santillana, M. & Brownstein, J. S. Antibiotic resistance increases with local temperature. Nat. Clim. Chang 8, 510 (2018).
Krumperman, P. H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol 46, 165–170 (1983).
The Center for Disease Dynamics Economics & Policy. ResistanceMap: Antibiotic resistance. https://resistancemap.cddep.org/AntibioticResistance.php (2018).
The Center for Disease Dynamics Economics & Policy. ResistanceMap: Antibiotic use. https://resistancemap.cddep.org/AntibioticUse.php (2018).
Paun, A., Acton, L. & Chan, W. S. Fragile Planet. Scoring Climate Risks Around the World (HSBC Global Research, 2018).
Hendriksen, R. S. et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat. Commun. 10, 1124 (2019).
Van Boeckel, T. P. et al. Global trends in antimicrobial resistance in animals in low- and middle- income countries. Science 365, 1266 (2019).
Nadimpalli, M. et al. Combating global antibiotic resistance: emerging one health concerns in lower- and middle-income countries. Clin. Infect. Dis. 66, 963–969 (2018).
Collingon, P., Beggs, J. P., Walsh, T. R., Gandra, S. & Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: a univariate and multivariate analysis. Lan. Plan. Health 2, e398–e405 (2018).
Baker-Austin, C. et al. Emerging Vibrio risk at high latitudes in response to ocean warming. Nat. Clim. Change 3, 73–77 (2013).
Ashrafi, R. et al. Broad thermal tolerance is negatively correlated with virulence in an opportunistic bacterial pathogen. Evol. Appl 11, 1700–1714 (2018).
Dittmar, J., Janssen, H., Kuske, A., Kurtz, J. & Scharsack, J. P. Heat and immunity: an experimental heat wave alters immune functions in three-spined sticklebacks (Gasterosteus aculeatus). J. Anim. Ecol. 83, 744–757 (2014).
O’Gorman, E. J. et al. Temperature effects on fish production across a natural thermal gradient. Glob. Chang. Biol. 22, 3206–3322 (2016).
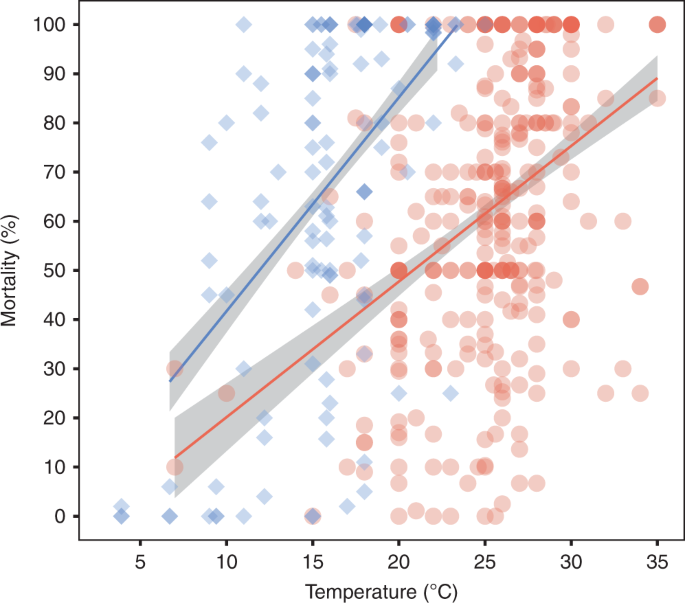
Leung, T. L. F. & Bates, A. E. More rapid and severe disease outbreaks for aquaculture at the tropics: implications for food security. J. Appl. Ecol. 50, 215–222 (2013).
Redshaw, C. H., Stahl-Timmins, W. M., Fleming, L. E., Davidson, I. & Depledge, M. H. Potential changes in disease patterns and pharmaceutical use in response to climate change. J. Toxicol. Environ. Health B Crit. Rev. 16, 285–320 (2013).
Interagency Coordination Group on Antimicrobial Resistance (IACG). No time to wait: securing the future from drug-resistant infections (2019).
European Council, 2001a Directive 2001/82/EC of the European Parliament and of the Council of 6th November 2001 on the Community code relating to veterinary medicinal products. Off. J. Eur. Community L-311, 1–66 (2004).
Henriksson, P. J. G., Troell, M. & Rico, A. Antimicrobial use in aquaculture: some complementing facts. Proc. Natl Acad. Sci. USA 112, E3317 (2015).
Rico, A. et al. Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia. Aquaculture 412-413, 231–243 (2013).
Shariff, M., Nagaraj, G., Chua, F. H. C. & Wang, Y. G. The use of chemicals in aquaculture in Malaysia and Singapore. Use of Chemicals in Aquaculture in Asia (edited by J. R. Arthur, C. R. Larilla-Pitogo & R. P. Subasinghe), Pp. 127–140. Proceedings of the Meeting on the Use of Chemicals in Aquaculture in Asia, 20–22 May 1996, (Southeast Asian Fisheries Development Center Aquaculture Department, Tigbauan, Iloilo, Philippines, 2000).
Bondad-Reantaso, M. G., Arthur, J. R. & Subasinghe, R. P., eds. Improving Biosecurity through Prudent and Responsible Use of Veterinary Medicines in Aquatic Food Production. FAO Fisheries and Aquaculture Technical Paper. No. 547. Rome, FAO. 207 pp (2012).
Liu, X., Steele, J. C. & Meng, X. Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: a review. Environ. Pollut. 223, 161–169 (2017).
Samuelsen, O. B., Torsvik, V. & Evik, A. Long-range change in oxytetracycline concentration and bacterial resistance towards oxytetracycline in a fish farm sediment after medication. Sci. Tot. Env 114, 25–36 (1992).
Tamminem, M. et al. Tetracycline resistance genes persist at aquaculture farms in the absence of selection pressure. Environ. Sci. Technol. 45, 386–391 (2011).
Hatosy, S. M. & Martiny, A. C. The Ocean as a global reservoir of antibiotic resistance genes. Appl. Environ. Microbiol. 81, 7593–7599 (2015).
Bentzon‐Tilia, M., Sonnenschein, E. C. & Gram, L. Monitoring and managing microbes in aquaculture – Towards a sustainable industry. Micro. Biotechnol. 9, 576–584 (2016).
Aubin, J. et al. Implementing ecological intensification in fish farming: definition and principles from contrasting experiences. Rev. Aquacult 11, 149–167 (2019).
Meek, R. W., Vyas, H. & Piddock, L. J. V. Nonmedical uses of antibiotics: time to restrict their use? PLoS Biol. 13, e1002266 (2015).
Petersen, A., Andersen, J. S., Kaewmak, T., Somsiri, T. & Dalsgaard, A. Impact of integrated fish farming on antimicrobial resistance in a pond environment. Appl. Environ. Microbiol. 68, 6036–6042 (2002).
Ahmed, N., Bunting, S. W., Rahman, S. & Garforth, C. J. Community-based climate change adaptation strategies for integrated prawn–fish–rice farming in Bangladesh to promote social-ecological resilience. Rev. Aquacult 6, 20–35 (2014).
Shifflett, S. D., Culbreth, A., Hazel, D., Daniels, H. & Nichols, E. G. Coupling aquaculture with forest plantations for food, energy, and water resiliency. Sci. Total. Environ. 571, 1262–1270 (2016).
Brudeseth, B. E. et al. Status and future perspectives of vaccines for industrialised fin-fish farming. Fish. Shellfish Immunol. 35, 1759–1768 (2013).
Reverter, M., Bontemps, N., Lecchini, D., Banaigs, B. & Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. Aquaculture 433, 50–61 (2014).
Hoseinifar, S. H., Sun, Y. Z., Wang, A. & Zhou, Z. Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front. Microbiol. 9, 2429 (2018).
Naylor, R. L. et al. Feeding aquaculture in an era of finite resources. PNAS 106, 15103–15110 (2009).
Caruso, D. et al. Traditional pharmacopeia in small scale freshwater farmers in West Java, Indonesia: an ethnoveterinary approach. Aquaculture 416-417, 334–345 (2013).
Caruso, D. et al. Herbal therapy in small-scale aquaculture: an ethnobotanic approach in North Vietnam and Central Java, Indonesia. In: 9th Symposium on Diseases in Asian Aquaculture (DAA9), 24-28 November 2014, (Ho Chi Minh City, 2014).
Tang, K. L. et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet. Health 1, e316–e327 (2017).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097 (2009).
Gurevitch, J., Koricheva, J., Nakagawa, S. & Stewart, G. Meta-analysis and the science of research synthesis. Nature 555, 175–182 (2018).
Arnold, T. W. Uninformative parameters and using Akaike’s Information Criterion. J. Wildlife Manag. 74, 1175–1178 (2010).
Source: Ecology - nature.com



