Schimel, D. S. Terrestrial ecosystems and the carbon-cycle. Glob. Change Biol. 1, 77–91, https://doi.org/10.1111/j.1365-2486.1995.tb00008.x. (1995).
Raich, J. W. & Schlesinger, W. H. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 44, 81–99, https://doi.org/10.1034/j.1600-0889.1992.t01-1-00001.x (1992).
Schlesinger, W. H. & Andrews, J. A. Soil respiration and the global carbon cycle. Biogeochemistry 48, 7–20, https://doi.org/10.1023/A:1006247623877 (2000).
Cox, P. M., Betts, R. A., Jones, C. D., Spall, S. A. & Totterdell, I. J. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408, 184–187, https://doi.org/10.1038/35041539 (2000).
Giardina, C. P. & Ryan, M. G. Evidence that decomposition rates of organic carbon in mineral soil do not vary with temperature. Nature 404, 858–861, https://doi.org/10.1038/35009076 (2000).
Luo, Y., Wan, S., Hui, D. & Wallace, L. L. Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413, 622–625, https://doi.org/10.1038/35098065 (2001).
Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 38, 425–448, https://doi.org/10.1016/j.soilbio.2005.08.020 (2006).
Bond-Lamberty, B. & Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 464, 579–582, https://doi.org/10.1038/nature08930 (2010).
Lloyd, J. & Taylor, J. A. On the temperature dependence of soil respiration. Funct. Ecol 8, 315–323, https://doi.org/10.2307/2389824 (1994).
Fang, C. & Moncrieff, J. B. A model for soil CO2 production and transport 1: Model development. Agric. For. Meteorol 95, 225–236, https://doi.org/10.1016/S0168-1923(99)00036-2 (1999).
Jensen, L. S. et al. Soil surface CO2 flux as an index of soil respiration in situ: A comparison of two chamber methods. Soil Biol. Biochem. 28, 1297–1306, https://doi.org/10.1016/S0038-0717(96)00136-8 (1996).
Selsted, M. B. et al. Soil respiration is stimulated by elevated CO2 and reduced by summer drought: three years of measurements in a multifactor ecosystem manipulation experiment in a temperate heathland (CLIMAITE). Glob. Change Biol. 18, 1216–1230, https://doi.org/10.1111/j.1365-2486.2011.02634.x (2012).
Schlesinger, W. H. The formation of caliche in soils of the Mojave Desert, California. Geochim. Cosmochim. Acta 49, 57–66, https://doi.org/10.1016/0016-7037(85)90191-7 (1985).
Schlesinger, W. H. Biogeochemistry: an analysis of global change. (Academic press, 1997).
Wang, Y. G., Hong, Z. & Yan, L. Spatial heterogeneity of soil moisture, microbial biomass carbon and soil respiration at stand scale of an arid scrubland. Environmental Earth Sciences 70, 3217–3224, https://doi.org/10.1007/s12665-013-2386-z (2013).
Li, Y., Hou, C., Song, C. & Guo, Y. Seasonal changes in the contribution of root respiration to total soil respiration in a freshwater marsh in Sanjiang Plain, Northeast China. Environmental Earth Sciences 75, 848, https://doi.org/10.1007/s12665-016-5592-7 (2016).
Emmerich, W. E. Carbon dioxide fluxes in a semiarid environment with high carbonate soils. Agric. For. Meteorol 116, 91–102, https://doi.org/10.1016/S0168-1923(02)00231-9 (2003).
Inglima, I. et al. Precipitation pulses enhance respiration of Mediterranean ecosystems: the balance between organic and inorganic components of increased soil CO2 efflux. Glob. Change Biol. 15, 1289–1301, https://doi.org/10.1111/j.1365-2486.2008.01793.x (2009).
Mielnick, P., Dugas, W. A., Mitchell, K. & Havstad, K. Long-term measurements of CO2 flux and evapotranspiration in a Chihuahuan desert grassland. Journal of Arid Environments 60, 423–436, https://doi.org/10.1016/j.jaridenv.2004.06.001 (2005).
Serrano-Ortiz, P. et al. Hidden, abiotic CO2 flows and gaseous reservoirs in the terrestrial carbon cycle: Review and perspectives. Agric. For. Meteorol 150, 321–329, https://doi.org/10.1016/j.agrformet.2010.01.002 (2010).
Stone, R. Have desert researchers discovered a hidden loop in the carbon cycle? Science 320, 1409–1410, https://doi.org/10.1126/science.320.5882.1409 (2008).
Ball, B. A., Virginia, R. A., Barrett, J. E., Parsons, A. N. & Wall, D. H. Interactions between physical and biotic factors influence CO2 flux in Antarctic dry valley soils. Soil Biol. Biochem. 41, 1510–1517, https://doi.org/10.1016/j.soilbio.2009.04.011 (2009).
Kowalski, A. S. et al. Can flux tower research neglect geochemical CO2 exchange? Agric. For. Meteorol 148, 1045–1054, https://doi.org/10.1016/j.agrformet.2008.02.004 (2008).
Serrano-Ortiz, P. et al. Interannual CO2 exchange of a sparse Mediterranean shrubland on a carbonaceous substrate. Journal of Geophysical Research: Biogeosciences 114, https://doi.org/10.1029/2009JG000983 (2009).
Jasoni, R. L., Smith, S. D. & Arnone, J. A. Net ecosystem CO2 exchange in Mojave Desert shrublands during the eighth year of exposure to elevated CO2. Glob. Change Biol. 11, 749–756, https://doi.org/10.1111/j.1365-2486.2005.00948.x (2005).
Wohlfahrt, G., Fenstermaker, L. F. & Arnone, J. A. Large annual net ecosystem CO2 uptake of a Mojave Desert ecosystem. Glob. Change Biol. 14, 1475–1487, https://doi.org/10.1111/j.1365-2486.2008.01593.x (2008).
Xie, J., Li, Y., Zhai, C., Li, C. & Lan, Z. CO2 absorption by alkaline soils and its implication to the global carbon cycle. Environmental Geology 56, 953–961, https://doi.org/10.1007/s00254-008-1197-0 (2009).
Stevenson, B. A. & Verburg, P. S. J. Effluxed CO2–13Cfrom sterilized and unsterilized treatments of a calcareous soil. Soil Biol. Biochem. 38, 1727–1733, https://doi.org/10.1016/j.soilbio.2005.11.028 (2006).
Shanhun, F. L., Almond, P. C., Clough, T. J. & Smith, C. M. S. Abiotic processes dominate CO2 fluxes in Antarctic soils. Soil Biol. Biochem. 53, 99–111, https://doi.org/10.1016/j.soilbio.2012.04.027 (2012).
Fa, K. et al. Underestimation of soil respiration in a desert ecosystem. CATENA 162, 23–28, https://doi.org/10.1016/j.catena.2017.11.019 (2018).
Schlesinger, W. H. An evaluation of abiotic carbon sinks in deserts. Glob. Change Biol. 23, 25–27, https://doi.org/10.1111/gcb.13336 (2017).
Schlesinger, W. H., Belnap, J. & Marion, G. On carbon sequestration in desert ecosystems. Glob. Change Biol. 15, 1488–1490, https://doi.org/10.1111/j.1365-2486.2008.01763.x (2009).
Fa, K. Y. et al. Patterns and possible mechanisms of soil CO2 uptake in sandy soil. Science of the Total Environment 544, 587–594, https://doi.org/10.1016/j.scitotenv.2015.11.163 (2016).
Angert, A. et al. Using O2 to study the relationships between soil CO2 efflux and soil respiration. Biogeosciences Discussions 11, 12039–12068, https://doi.org/10.5194/bg-12-2089-2015 (2014).
Huang, J., Yu, H., Guan, X., Wang, G. & Guo, R. Accelerated dryland expansion under climate change. Nature Climate Change 6, 166–171, https://doi.org/10.1038/nclimate2837 (2015).
Chapin, F. S., Randerson, J. T., McGuire, A. D., Foley, J. A. & Field, C. B. Changing feedbacks in the climate-biosphere system. Front. Ecol. Environ. 6, 313–320, https://doi.org/10.1890/080005 (2008).
Biasutti, M. Climate change: Future rise in rain inequality. Nat. Geosci. 6, 337–338, https://doi.org/10.1038/ngeo1814 (2013).
Borken, W. & Matzner, E. Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Glob. Change Biol. 15, 808–824, https://doi.org/10.1111/j.1365-2486.2008.01681.x (2009).
Huang, J., Yu, H., Dai, A., Wei, Y. & Kang, L. Drylands face potential threat under 2 °C global warming target. Nature Climate Change https://doi.org/10.1038/nclimate3275 (2017).
Wang, Y., Wang, Z. & Li, Y. Storage/turnover rate of inorganic carbon and its dissolvable part in the profile of saline/alkaline soils. PLoS One 8, e82029, https://doi.org/10.1371/journal.pone.0082029 (2013).
Xu, G.-Q., Yu, D.-D. & Li, Y. Patterns of biomass allocation in Haloxylon persicum woodlands and their understory herbaceous layer along a groundwater depth gradient. Forest Ecology and Management 395, 37–47 (2017).
Maestre, F. T. & Cortina, J. Small-scale spatial variation in soil CO2 efflux in a Mediterranean semiarid steppe. Appl. Soil Ecol 23, 199–209, https://doi.org/10.1016/S0929-1393(03)00050-7 (2003).
Pen-Mouratov, S., Rakhimbaev, M. & Steinberger, Y. Spatio-temporal effect on soil respiration in fine-scale patches in a desert ecosystem. Pedosphere 16, 1–9, https://doi.org/10.1016/S1002-0160(06)60019-2 (2006).
Ma, J., Wang, Z. Y., Stevenson, B. A., Zheng, X. J. & Li, Y. An inorganic CO2 diffusion and dissolution process explains negative CO2 fluxes in saline/alkaline soils. Sci Rep 3, 2025, https://doi.org/10.1038/srep02025 (2013).
Ruehr, N. K., Knohl, A. & Buchmann, N. Environmental variables controlling soil respiration on diurnal, seasonal and annual time-scales in a mixed mountain forest in Switzerland. Biogeochemistry 98, 153–170, https://doi.org/10.1007/s10533-009-9383-z (2010).
Nelson, D. W. & Sommers, L. E. Total carbon, organic carbon, and organic matter. Methods of soil analysis. Part 3-chemical methods. 3, 961–1010, https://doi.org/10.2136/sssabookser5.3.c34 (1996).
Sherrod, L. A., Dunn, G., Peterson, G. A. & Kolberg, R. L. Inorganic carbon analysis by modified pressure-calcimeter method. Soil Sci. Soc. Am. J 66, 299–305, https://doi.org/10.2136/sssaj2002.2990 (2002).
Luo, Y. & Zhou, X. Soil respiration and the environment. (San Diego, CA, USA: Academic Press/Elsevier., 2006).
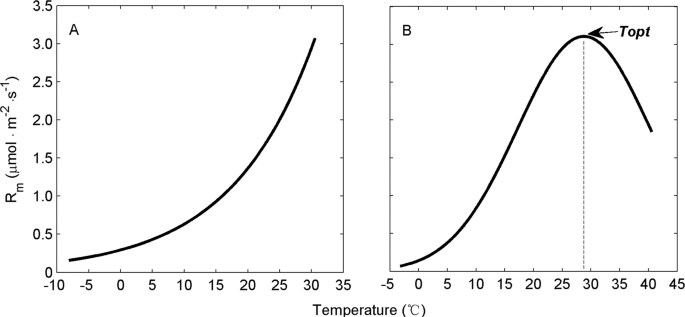
Richardson, J., Chatterjee, A. & Jenerette, G. D. Optimum temperatures for soil respiration along a semi-arid elevation gradient in southern California. Soil Biol. Biochem. 46, 89–95, https://doi.org/10.1016/j.soilbio.2011.11.008 (2012).
Davidson, E. A., Janssens, I. A. & Luo, Y. Q. On the variability of respiration in terrestrial ecosystems: moving beyond Q10. Glob. Change Biol. 12, 154–164 (2006).
Davidson, E. A. & Janssens, I. A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440, 165–173, https://doi.org/10.1038/Nature04514 (2006).
Flanagan, P. & Veum, A. Relationships between respiration, weight loss, temperature and moisture in organic residues on tundra. Soil organisms and decomposition in tundra, 249–277 (1974).
Xie, J.-B., Xu, G.-Q., Cao, X., Wang, Z.-Y. & Li, Y. When the classical reaction norm is corrected by body size. Perspectives in Plant Ecology Evolution & Systematics 17, 454–466, https://doi.org/10.1016/j.ppees.2015.09.007 (2015).
Li, Y., Wang, Y.-G., Houghton, R. A. & Tang, L.-S. Hidden carbon sink beneath desert. Geophysical Research Letters 42, 5880–5887, https://doi.org/10.1002/2015GL064222 (2015).
Datta, R., Vranová, V., Pavelka, M., Rejšek, K. & Formánek, P. Effect of soil sieving on respiration induced by low-molecular-weight substrates. Int. Agrophys. 28, 119–124, https://doi.org/10.2478/intag-2013-0034 (2014).
Six, J., Conant, R. T., Paul, E. A. & Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant and soil 241, 155–176, https://doi.org/10.1023/a:1016125726789 (2002).
Xu, H. & Li, Y. Water-use strategy of three central Asian desert shrubs and their responses to rain pulse events. Plant and soil 285, 5–17, https://doi.org/10.1007/s11104-005-5108-9 (2006).
Ji, F., Wu, Z., Huang, J. & Chassignet, E. P. Evolution of land surface air temperature trend. Nature Climate Change 4, 462–466, https://doi.org/10.1038/nclimate2223 (2014).
Were, A. et al. Ventilation of subterranean CO2 and Eddy covariance incongruities over carbonate ecosystems. Biogeosciences 7, 859–867, https://doi.org/10.5194/bg-7-859-2010 (2010).
Soper, F. M., McCalley, C. K., Sparks, K. & Sparks, J. P. Soil carbon dioxide emissions from the Mojave desert: Isotopic evidence for a carbonate source. Geophysical Research Letters 44, 245–251, https://doi.org/10.1002/2016gl071198 (2017).
Parsons, A. N., Barrett, J. E., Wall, D. H. & Virginia, R. A. Soil carbon dioxide flux in Antarctic dry valley ecosystems. Ecosystems 7, 286–295, https://doi.org/10.1007/s10021-003-0132-1 (2004).
Roland, M. et al. Atmospheric turbulence triggers pronounced diel pattern in karst carbonate geochemistry. Biogeosciences Discussions 10, 1207–1227, https://doi.org/10.5194/bg-10-5009-2013 (2013).
Davidson, E. A., Belk, E. & Boone, R. D. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Change Biol. 4, 217–227, https://doi.org/10.1046/j.1365-2486.1998.00128.x (1998).
Fang, C. & Moncrieff, J. B. The dependence of soil CO2 efflux on temperature. Soil Biol. Biochem. 33, 155–165, https://doi.org/10.1016/S0038-0717(00)00125-5 (2001).
Cable, J. M. et al. The temperature responses of soil respiration in deserts: a seven desert synthesis. Biogeochemistry 103, 71–90, https://doi.org/10.1007/s10533-010-9448-z (2011).
Source: Ecology - nature.com


