Green, D. G. & Sadedin, S. Interactions matter—complexity in landscapes and ecosystems. Ecol Complex 2, 117–130 (2005).
Smith, H. Light Quality, Photoperception, and Plant Strategy. Ann Rev Plant Physio 33, 481–518 (1982).
Baldwin, I. T. & Schultz, J. C. Rapid Changes in Tree Leaf Chemistry Induced by Damage: Evidence for Communication between Plants. Science 221, 277–279 (1983).
Rhoades, D. F. Responses of Alder and Willow to Attack by Tent Caterpillars and Webworms: Evidence for Pheromonal Sensitivity of Willows in Plant Resistance to Insects (ed. Hedin, P. A.) 55–68 (American Chemical Society, 1983).
Keuskamp, D. H., Sasidharan, R. & Pierik, R. Physiological regulation and functional significance of shade avoidance responses to neighbors. Plant Signal Behav 5, 655–662 (2010).
de Wit, M. et al. Plant neighbor detection through touching leaf tips precedes phytochrome signals. PNAS 20, 1–6 (2012).
Elhakeem, A., Markovic, D., Broberg, A., Anten, N. P. R. & Ninkovic, V. Aboveground mechanical stimuli affect belowground plant-plant communication. PLoS One 13, e0195646 (2018).
Markovic, D., Nikolic, N., Glinwood, R., Seisenbaeva, G. & Ninkovic, V. Plant Responses to Brief Touching: A Mechanism for Early Neighbour Detection? PLoS One 9, 1–19 (2016).
Markovic, D. et al. Airborne signals synchronize the defenses of neighboring plants in response to touch. J Exp Bot 70, 691–700 (2019).
Appel, H. M. & Cocroft, R. B. Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia 175, 1257–1266 (2014).
Babikova, Z. et al. Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack. Ecol Lett 16, 835–843 (2013).
Biedrzycki, M. L., Jilany, T. A., Dudley, S. A. & Bais, H. P. Root exudates mediate kin recognition in plants. Comm Integr Biol 3, 28–35 (2010).
Tumlinson, J. H. The Importance of Volatile Organic Compounds in Ecosystem Functioning. J Chem Ecol 40, 212–213 (2014).
Heil, M. & Karban, R. Explaining evolution of plant communication by airborne signals. Trends Ecol Evol 25, 137–144 (2010).
Farag, M. A., Zhang, H. & Ryu, C. M. Dynamic Chemical Communication between Plants and Bacteria through Airborne Signals: Induced Resistance by Bacterial Volatiles. J Chem Ecol 39, 1007–1018 (2013).
Thomas, F. et al. Waterborne Signaling Primes the Expression of Elicitor-Induced Genes and Buffers the Oxidative Responses in the Brown Alga Laminaria digitata. PLoS ONE 6, 1–12 (2011).
Ditengou, F. A. et al. Volatile signalling by sesquiterpenes from ectomycorrhizal fungi reprogrammes root architecture. Nat Commun 6, 1–9 (2015).
Abedesin, F. et al. Emission of volatile organic compounds from petunia flowers is facilitated by an ABC transporter. Science 30, 1386–1388 (2017).
Widhalm, J. R., Jaini, R., Morgan, J. A. & Dudareva, N. Rethinking how volatiles are released from plant cells. Trends Plant Sci 20, 545–550 (2015).
de Moraes, C. M., Mescher, M. C. & Tumlinson, J. H. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410, 577–580 (2001).
Karban, R., Wetzel, W. C., Shiojiri, K., Pezzola, E. & Blande, J. D. Geographic dialects in volatile communication between sagebrush individuals. Ecology 97, 2917–2924 (2016).
Kegge, W. et al. Red:far-red light conditions affect the emission of volatile organic compounds from barley (Hordeum vulgare), leading to altered biomass allocation in neighbouring plants. Ann Bot-London 115, 961–970 (2015).
Ninkovic, V., Markovic, D. & Dahlin, I. Decoding neighbour volatiles in preparation for future competition and implications for tritrophic interactions. Perspect Plant Ecol 23, 11–17 (2016).
Runyon, J. B., Mescher, M. C. & De Moraes, C. M. Volatile chemical cues guide host location and host selection by parasitic plants. Science 313, 1964–1967 (2006).
Scala, A., Allmann, S., Mirabella, R., Haring, M. A. & Schuurink, R. C. Green Leaf Volatiles: A Plant’s Multifunctional Weapon against Herbivores and Pathogens. Int J Mol Sci 14, 17781–17811 (2013).
Ninkovic, V. Volatile communication between barley plants affects biomass allocation. J Exp Bot 54, 1931–1939 (2003).
Caparrotta, S. et al. Induction of priming by salt stress in neighboring plants. Environ Exp Bot 147, 261–270 (2018).
Karban, R., Shiojiri, K., Huntzinger, M. & Mc Call, A. C. Damage-induced resistance in sagebrush: volatiles are key to intra-and interplant communication. Ecology 87, 922–930 (2006).
Dahlin, I., Rubene, D., Glinwood, R. & Ninkovic, V. Pest suppression in cultivar mixtures is influenced by neighbor-specific plant–plant communication. Ecol Appl 28, 2187–2196 (2018).
Ninkovic, V., Al Abassi, S., Ahmed, E., Glinwood, R. & Pettersson, J. Effect of within-species plant genotype mixing on habitat preference of a polyphagous insect predator. Oecologia 166, 391–400 (2011).
Lazebnik, J. Jack Pine Signalling and Responses to Herbivory. MS thesis, University of Alberta. 103 pp. (2012).
Marino, P., Raguso, R. & Goffinet, B. The ecology and evolution of fly dispersed dung mosses (Family Splachnaceae): Manipulating insect behaviour through odour and visual cues. Symbiosis 47, 61–76 (2009).
Rosenstiel, T. N., Shortlidge, E. E., Melnychenko, A. N., Pankow, J. F. & Eppley, S. M. Sex-specific volatile compounds influence microarthropod-mediated fertilization of moss. Nature 489, 431–433 (2012).
Rydin, H. Competition among bryophytes. Advances in bryology 6, 135–168 (1997).
Van der Hoeven, E. C., Korporaal, M. & Van Gestel, E. Effects of simulated shade on growth, morphology and competitive interactions in two pleurocarpous mosses. J Bryol 20, 301–310 (1998).
Ballaré, C. L. & Pierik, R. The shade-avoidance syndrome: Multiple signals and ecological consequences. Plant Cell Environ 11, 2530–2543 (2017).
McCuaig, B., Dufour, S. C., Raguso, R. A., Bhatt, A. P. & Marino, P. Structural changes in plastids of developing Splachnum ampullaceum sporophytes and relationship to odour production. Plant Biology 17, 466–473 (2014).
Chen, F. et al. Terpenoid secondary metabolites in bryophytes: chemical diversity, biosynthesis and biological functions. Crit Rev Plant Sci 37, 210–231 (2018).
Ramel, F. et al. Light-Induced Acclimation of the Arabidopsis chlorina1 Mutant to Singlet Oxygen. Plant Cell 25, 1445–1462 (2013).
Kato-Noguchi, H. & Seki, T. Allelopathy of the moss Rhynchostegium pallidifolium and 3-hydroxy-β-ionone. Plant Signal Behav 5, 702–704 (2010).
Engelberth, J., Alborn, H. T., Schmelz, E. A. & Tumlinson, J. H. Airborne signals prime plants against insect herbivore attack. PNAS 10, 1781–1785 (2004).
Kigathi, R. N., Weisser, W. W., Reichelt, M., Gershenzon, J. & Unsicker, S. B. Plant volatile emission depends on the species composition of the neighboring plant community. BMC Plant Biol 19, 58 (2019).
Quintana-Rodriguez, E. et al. Plant volatiles cause direct, induced and associational resistance in common bean to the fungal pathogen Colletotrichum lindemuthianum. J Ecol 103, 250–260 (2015).
Ninkovic, V. et al. Volatile Exchange between Undamaged Plants – a New Mechanism Affecting Insect Orientation in Intercropping. PLoS One 8, e69431, https://doi.org/10.1371/journal.pone.0069431 (2013).
Vucetic, A. et al. Volatile interaction between undamaged plants affects tritrophic interactions through changed plant volatile emission. Plant Signal Behav 9, e29517 (2014).
Cronberg, N. Clonal structure and fertility in a sympatric population of the peat mosses Sphagnum rubellum and Sphagnum capillifolium. Can J Botany 74, 1375–1385 (1996).
Rydin, H. Competition and niche separation in Sphagnum. Can J Botany 64, 1817–1824 (1986).
Mälson, K. & Rydin, H. Competitive hierarchy, but no competitive exclusions in experiments with rich fen bryophytes. J Bryol 31, 41–45 (2009).
Asakawa, Y. Liverworts-Potential Source of Medicinal Compounds. Curr Pharm Design 14, 3067–3088 (2008).
Asakawa, Y., Ludwiczuk, A. & Nagashima, F. Phytochemical and biological studies of bryophytes. Phytochemistry 91, 52–80 (2013).
Gupta, S. K., Sharma, A. & Moktan, S. A review on some species of Marchantia with reference to distribution, characterization and importance. Word journal of pharmacy and pharmaceutical sciences 4, 1576–1588 (2015).
Valarezo, E. et al. Essential Oil Constituents of Mosses Species from Ecuador. J Essent Oil Bear Pl 21, 189–197 (2018).
Wu, C.-L. Chemosystematic Correlations of Taiwanese Hepaticae. J Chin Chem Soc_Taip 39, 655–667 (1992).
Glime, J. M. & Rohwer, F. The comparative effects of ethylene and l-amino-cyclopropanel- carboxylic acid on two species of Fontinalis. J Bryol 12, 611–616 (1983).
Yasumura, Y., Pierik, R., Fricker, M. D., Voesenek, L. A. C. J. & Harberd, N. P. Studies of Physcomitrella patens reveal that ethylene mediated submergence responses arose relatively early in land-plant evolution. Plant J 72, 947–959 (2012).
Thomas, R. J., Harrison, M. A., Taylor, J. & Kaufman, P. B. Endogenous auxin and ethylene in Pellia (Bryophyta). Plant Physiol 73, 395–397 (1983).
Jackson, M. B. Ethylene-promoted Elongation: an Adaptation to Submergence Stress. Ann Bot-London 101, 229–248 (2008).
Pierik, R., Visser, E. J. W., De Kroon, H. & Voesenek, L. A. C. J. Ethylene is required in tobacco to successfully compete with proximate neighbours. Plant Cell Environ 26, 1229–1234 (2003).
Pierik, R., Cuppens, M. L. C., Voesenek, L. A. C. J. & Visser, E. J. W. Interactions between Ethylene and Gibberellins in Phytochrome-Mediated Shade Avoidance Responses in Tobacco. Plant Physiol 136, 2928–2936 (2004).
Kegge, W., Weldegergis, B. T., Soler, R., Eijk, M. V. V. & Dicke, M. Canopy light cues affect emission of constitutive and methyl jasmonate-induced volatile organic compounds in Arabidopsis thaliana. New Phytol 200, 861–874 (2013).
Jägerbrand, A. K. & During, H. J. Effects of simulated shade on growth, number of branches and biomass in Hylocomium splendens and Racomitrium lanuginosum. Lindbergia 30, 117–124 (2006).
Possart, A. & Hiltbrunner, A. An Evolutionarily Conserved Signaling Mechanism Mediates Far-Red Light Responses in Land Plants. The Plant Cell 25, 102–114 (2013).
van Hinsberg, A. & van Tienderen, P. Variation in growth form in relation to spectral light quality (red/far-red ratio) in Plantago lanceolata L. in sun and shade populations. Oecologia 111, 452–459 (1997).
Más, P., Devlin, P. F., Panda, S. & Kay, S. A. Functional interaction of phytochrome B and cryptochrome 2. Nature 408, 207–211 (2000).
Pedmale, U. V. et al. Cryptochromes Interact Directly with PIFs to Control Plant Growth in Limiting Blue Light. Cell 164, 1–13 (2016).
Hanyu, H. & Shoji, K. Effects of Blue Light and Red Light on Kidney Bean Plants Grown under Combined Radiation from Narrow-Band Light Sources. Environ Control Biol 38, 13–24 (2000).
Runkle, E. S. & Heins, R. D. Specific Functions of Red, Far Red, and Blue Light in Flowering and Stem Extension of Long-day Plants. J Am Soc Hortic Sci 126, 275–282 (2001).
Rydin, H. & Barber, K. E. Long term and fine scale coexistence of closely related species. Folia Geobot 36, 53–61 (2001).
Gunnarson, U., Shaw, A. J. & Lӧnn, M. Local‐scale genetic structure in the peatmoss Sphagnum fuscum. Mol Ecol 16, 305–312 (2007).
Rydin, H. Interspecific Competition between Sphagnum Mosses on a Raised Bog. Oikos 66, 413–423 (1993).
Stark, L. R. Ecology of desiccation tolerance in bryophytes: A conceptual framework and methodology. Bryologist 120, 129–164 (2017).
Bragazza, L. A climatic threshold triggers the die-off of peat mosses during an extreme heat wave. Glob Change Biol 14, 2688–2695 (2008).
Hájek, M., Horsák, M., Hájková, P. & Dítě, D. Habitat diversity of central European fens in relation to environmental gradients and an effort to standardise fen terminology in ecological studies. Perspect Plant Ecol 8, 97–114 (2006).
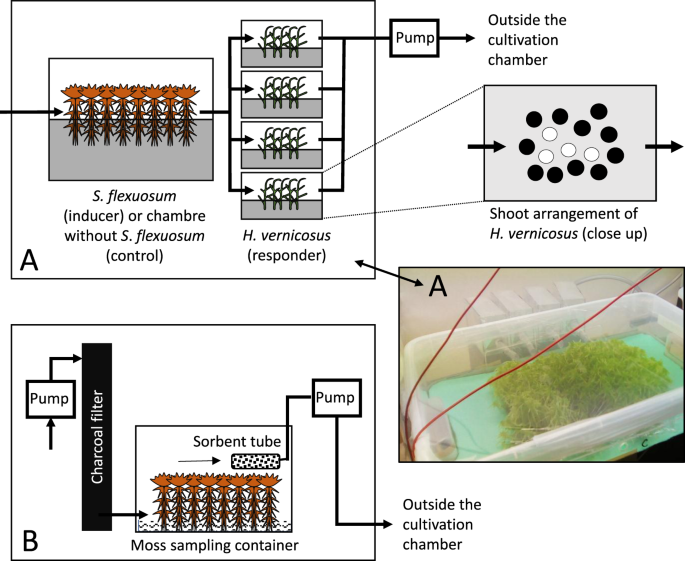
Štechová, T., Kučera, J. & Šmilauer, P. Factors affecting population size and vitality of Hamatocaulis vernicosus (Mitt.) Hedenäs (Calliergonaceae, Musci). Wetl Ecol Manag 20, 329–339 (2012).
Vicherová, E., Hájek, M. & Hájek, T. Calcium intolerance of fen mosses: Physiological evidence, effects of nutrient availability and successional drivers. Perspect Plant Ecol 17, 347–359 (2015).
Losvik, A. et al. Overexpression and down-regulation of barley lipoxygenase lox2.2 affects jasmonate-regulated genes and aphid fecundity. Int. J. Mol. Sci. 18, 2765 (2017).
Pinheiro, J., Bates, D., DebRoy, S. & Sarkar, D., R Core Team nlme: linear and nonlinear mixed effects models. [WWW document], https://cran.r-project.org/package=nlme (2017).
Source: Ecology - nature.com


