Hudson, P. & Greenman, J. Competition mediated by parasites: biological and theoretical progress. Trends Ecol. Evol. 13, 387–390 (1998).
Kohler, S. L. & Hoiland, W. K. Population regulation in an aquatic insect: the role of disease. Ecology 82, 2294–2305 (2001).
Lafferty, K. D. & Morris, A. K. Altered behavior of parasitized killifish increases susceptibility to predation by bird final hosts. Ecology 77, 1390–1397 (1996).
Holdo, R. M. et al. A disease-mediated trophic cascade in the Serengeti and its implications for ecosystem C. Plos Biol. 7, e1000210 (2009).
Brunner, F. S., Anaya-Rojas, J. M., Matthews, B. & Eizaguirre, C. Experimental evidence that parasites drive eco-evolutionary feedbacks. Proc. Natl. Acad. Sci. USA 114, 3678–3683 (2017).
Behringer, D. C., Butler, M. J. & Shields, J. D. Avoidance of disease by social lobsters. Nature 441, 421–421 (2006).
Woodworth, B. L. et al. Host population persistence in the face of introduced vector-borne diseases: Hawaii amakihi and avian malaria. Proc. Natl. Acad. Sci. USA 102, 1531–6 (2005).
Aeby, G. S. Behavioral and ecological relationships of a parasite and its hosts within a coral reef system. Pacific Sci. 45, 263–269 (1991).
King, G. & Zeng, L. Logistic Regression in Rare Events Data. Polit. Anal. 9, 137–163 (2001).
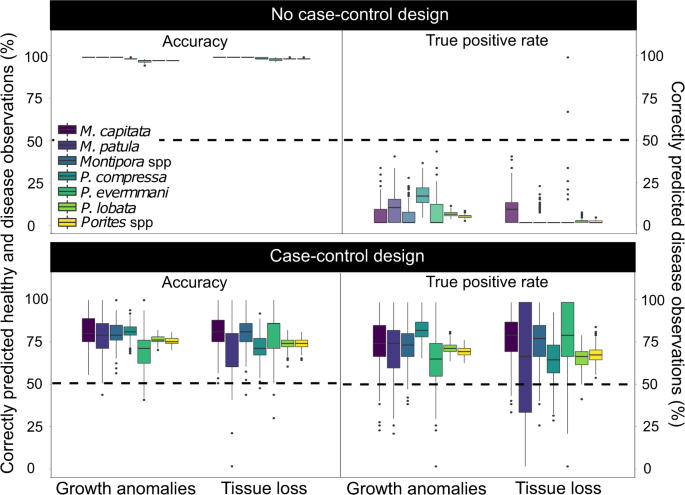
Lewallen, S. & Courtright, P. Epidemiology in practice: case-control studies. Community eye Heal. 11, 57–8 (1998).
Sutherland, K. P., Porter, J. W. & Torres, C. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar. Ecol. Prog. Ser. 266, 273–302 (2004).
Stimson, J. Ecological characterization of coral growth anomalies on Porites compressa in Hawai’i. Coral Reefs 30, 133–142 (2010).
Ruiz-Moreno, D. et al. Global coral disease prevalence associated with sea temperature anomalies and local factors. Dis. Aquat. Organ. 100, 249–261 (2012).
Palmer, C. V. & Baird, A. H. Coral tumor-like growth anomalies induce an immune response and reduce fecundity. Dis. Aquat. Organ. 130, 77–81 (2018).
Couch, C. S. et al. Spatial and temporal patterns of coral health and disease along leeward Hawai’i Island. Coral Reefs 33, 693–704 (2014).
Kaczmarsky, L. & Richardson, L. L. Do elevated nutrients and organic carbon on Philippine reefs increase the prevalence of coral disease? Coral Reefs 30, 253–257 (2011).
Aeby, G. S. et al. Growth anomalies on the coral genera Acropora and Porites are strongly associated with host density and human population size across the Indo-Pacific. Plos One 6, e16887 (2011).
Williams, G. J., Aeby, G. S., Cowie, R. O. M. & Davy, S. K. Predictive modeling of coral disease distribution within a reef system. PLoS One 5, e9264 (2010).
Aeby, G. S. et al. Emerging coral diseases in Kaneohe Bay, Oahu, Hawaii (USA): two major disease outbreaks of acute Montipora white syndrome. Dis. Aquat. Organ. 119, 189–198 (2016).
Brodnicke, O. B. et al. Unravelling the links between heat stress, bleaching and disease: fate of tabular corals following a combined disease and bleaching event. Coral Reefs 38, (2019).
Hobbs, J.-P. A., Frisch, A. J., Newman, S. J. & Wakefield, C. B. Selective Impact of Disease on Coral Communities: Outbreak of White Syndrome Causes Significant Total Mortality of Acropora Plate Corals. PLoS One 10, e0132528 (2015).
Precht, W. F., Gintert, B. E., Robbart, M. L., Fura, R. & van Woesik, R. Unprecedented Disease-Related Coral Mortality in Southeastern Florida. Sci. Rep. 6, 31374 (2016).
Walton, C. J., Hayes, N. K. & Gilliam, D. S. Impacts of a Regional, Multi-Year, Multi-Species Coral Disease Outbreak in Southeast Florida. Front. Mar. Sci. 5, (2018).
Sussman, M., Willis, B. L., Victor, S. & Bourne, D. G. Coral pathogens identified for White Syndrome (WS) epizootics in the Indo-Pacific. PLoS One 3, e2393 (2008).
Ben-Haim, Y. et al. Vibrio coralliilyticus sp. nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. Int. J. Syst. Evol. Microbiol. 53, 309–315 (2003).
Ushijima, B. et al. Vibrio coralliilyticus Strain OCN008 is an etiological agent of acute Montipora White Syndrome. Appl. Environ. Microbiol. 80, (2014).
Ushijima, B. et al. Mutation of the toxR or mshA genes from Vibrio coralliilyticus strain OCN014 reduces infection of the coral Acropora cytherea. Environ. Microbiol. 18, 4055–4067 (2016).
Bruno, J. F. et al. Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol. 5, e124 (2007).
Caldwell, J. M., Heron, S. F., Eakin, C. M. & Donahue, M. J. Satellite SST-Based Coral Disease Outbreak Predictions for the Hawaiian Archipelago. Remote Sens. 8, 93 (2016).
Heron, S. F. et al. Summer hot snaps and winter conditions: modelling white syndrome outbreaks on Great Barrier Reef corals. PLoS One 5, e12210 (2010).
Maynard, J. A. et al. Predicting outbreaks of a climate-driven coral disease in the Great Barrier Reef. Coral Reefs 30, 485–495 (2011).
Randall, C. J., Jordan-Garza, A. G., Muller, E. M. & Van Woesik, R. Relationships between the history of thermal stress and the relative risk of diseases of Caribbean corals. Ecology 95, 1981–1994 (2014).
Caldwell, J. M., Donahue, M. J. & Harvell, C. D. Host size and proximity to diseased neighbours drive the spread of a coral disease outbreak in Hawai’i. Proc. R. Soc. B 285, (2018).
Jolles, A. E., Sullivan, P., Alker, A. P. & Harvell, C. D. Disease Transmission of Aspergillosis in Sea Fans: Inferring Process from Spatial Pattern. Ecology 83, 2373–2378 (2002).
Bythell, J. C., Brown, B. E. & Kirkwood, T. B. L. Do reef corals age? Biol. Rev. 93, 1192–1202 (2018).
Bak, R. & Meesters, E. Coral population structure:the hidden information of colony size-frequency distributions. Mar. Ecol. Prog. Ser. 162, 301–306 (1998).
Meesters, E. H. et al. Colony size-frequency distributions of scleractinian coral populations: spatial and interspecific variation. Marine Ecology Progress Series 209, 43–54 (2001).
Jordán-Dahlgren, E., Jordán-Garza, A. G. & Rodríguez-Martínez, R. E. Coral disease prevalence estimation and sampling design. PeerJ 6, e6006 (2018).
Nugues, M. M., Smith, G. W., Hooidonk, R. J., Seabra, M. I. & Bak, R. P. M. Algal contact as a trigger for coral disease. Ecol. Lett. 7, 919–923 (2004).
Kaczmarsky, L. & Richardson, L. L. Transmission of growth anomalies between Indo-Pacific Porites corals. J. Invertebr. Pathol. 94, 218–221 (2007).
Yoshioka, R. M., Kim, C. J. S. S., Tracy, A. M., Most, R. & Harvell, C. D. Linking sewage pollution and water quality to spatial patterns of Porites lobata growth anomalies in Puako, Hawaii. Mar. Pollut. Bull. 104, 313–321 (2016).
Randall, C. J. & Van Woesik, R. Contemporary white-band disease in Caribbean corals driven by climate change. Nat. Clim. Chang. 5, 375–379 (2015).
Jones, R., Bowyer, J., Hoegh-Guldberg, O. & Blackall, L. L. Dynamics of a temperature-related coral disease outbreak. Mar. Ecol. Prog. Ser. 281, 63–77 (2004).
Lesser, M. P., Weis, V. M., Patterson, M. R. & Jokiel, P. L. Effects of morphology and water motion on carbon delivery and productivity in the reef coral, Pocillopora damicornis (Linnaeus): Diffusion barriers, inorganic carbon limitation, and biochemical plasticity. J. Exp. Mar. Bio. Ecol. 178, 153–179 (1994).
Work, T. M., Russell, R. & Aeby, G. S. S. Tissue loss (white syndrome) in the coral Montipora capitata is a dynamic disease with multiple host responses and potential causes. Proc. R. Soc. B. Biol. Sci. 279, 4334–4341 (2012).
Beurmann, S. et al. Pseudoalteromonas piratica strain OCN003 is a coral pathogen that causes a switch from chronic to acute Montipora white syndrome in Montipora capitata. PLoS One 12, e0188319 (2017).
Breslow, N. E. Statistics in Epidemiology: The Case-Control Study. J. Am. Stat. Assoc. 91, 14–28 (1996).
Venette, R. C., Moon, R. D. & Hutchison, W. D. Strategies and Statistics of Sampling for Rare Individuals. Annu. Rev. Entomol. 47, 143–174 (2002).
Balmaseda, A. et al. Index Cluster Study of Dengue Virus Infection in Nicaragua. Am. J. Trop. Med. Hyg. 83, 683–689 (2010).
Burnham, K., Anderson, D. & Laake, J. Estimation of Density from Line Transect Sampling of Biological Populations. Wildl. Monogr. 72, 3–202 (1980).
Caldwell, J. M. et al. Hawaiʻi Coral Disease database (HICORDIS): species-specific coral health data from across the Hawaiian archipelago. Data in Brief 8, 1054–1058 (2016).
Taylor, B. M. et al. Synchronous biological feedbacks in parrotfishes associated with pantropical coral bleaching. Glob. Chang. Biol. gcb.14909. https://doi.org/10.1111/gcb.14909 (2019).
Graham, N. A. J., Jennings, S., MacNeil, M. A., Mouillot, D. & Wilson, S. K. Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518, 94–97 (2015).
Stockwell, B., Jadloc, C., Abesamis, R., Alcala, A. & Russ, G. Trophic and benthic responses to no-take marine reserve protection in the Philippines. Mar. Ecol. Prog. Ser. 389, 1–15 (2009).
Douglas, B., Mächler, M., Bolker, B. & Walker, S. Fitting Linear Mixed-Effects Models Using lme4 | Bates | Journal of Statistical Software. J. Stat. Softw. 67, (2015).
Team, R. C. R: A Language and Environment for Statistical Computing. R Found. Stat. Comput. (2018).
Hobbs, J. & Frisch, A. Coral disease in the Indian Ocean: taxonomic susceptibility, spatial distribution and the role of host density on the prevalence of white syndrome. Dis. Aquat. Organ. 89, 1–8 (2010).
McClanahan, T. R., Weil, E. & Maina, J. Strong relationship between coral bleaching and growth anomalies in massive Porites. Glob. Chang. Biol 15, 1804–1816 (2009).
Aeby, G. S. et al. Patterns of Coral Disease across the Hawaiian Archipelago: Relating Disease to Environment. PLoS One 6, e20370 (2011).
Lamb, J. B. et al. Plastic waste associated with disease on coral reefs. Science 359, 460–462 (2018).
Raymundo, L. J., Halford, A. R., Maypa, A. P., Kerr, A. M. & Karl, D. M. Functionally diverse reef-fish communities ameliorate coral disease.
Garren, M., Smriga, S. & Azam, F. Gradients of coastal fish farm effluents and their effect on coral reef microbes. Environ. Microbiol 10, 2299–2312 (2008).
Lamb, J. B., Williamson, D. H., Russ, G. R. & Willis, B. L. Protected areas mitigate diseases of reef-building corals by reducing damage from fishing. Ecology 96, 150422141041004 (2015).
Bruno, J. F. et al. Nutrient enrichment can increase the severity of coral diseases. Ecol. Lett. 6, 1056–1061 (2003).
Sheridan, C., Baele, J. M., Kushmaro, A., Frejaville, Y. & Eeckhaut, I. Terrestrial runoff influences white syndrome prevalence in SW Madagascar. Mar. Environ. Res. 101, 44–51 (2014).
Haapkylä, J., Seymour, A. S., Trebilco, J. & Smith, D. Coral disease prevalence and coral health in the Wakatobi Marine Park, south-east Sulawesi, Indonesia. J. Mar. Biol. Assoc. UK 87, 403 (2007).
Heenan, A. et al. Ecological Monitoring 2012-2013 – reef fishes and benthic habitats of the main Hawaiian Islands, American Samoa, and Pacific Remote Island Areas, https://doi.org/10.13140/RG.2.1.4351.9122 (2014).
Wedding, L. M. et al. Advancing the integration of spatial data to map human and natural drivers on coral reefs. PLoS One 13, e0189792 (2018).
Frazier, A. G., Giambelluca, T. W., Diaz, H. F. & Needham, H. L. Comparison of geostatistical approaches to spatially interpolate monthyear rainfall for the Hawaiian Islands. Int. J. Climatol. 36, 1459–1470 (2016).
Lecky, J. H. Ecosystem Vulnerability and Mapping Cumulative Impacts on Hawaiian Reefs, https://doi.org/10.13140/RG.2.2.33194.52162 (University of Hawaii at Manoa, 2016).
Gelman, A. & Hill, J. Data analysis using regression and hierarchical/multilevel models. (Cambridge, 2007).
Source: Ecology - nature.com


