Rodriguez RJ Jr, JFW, Arnold AE, Redman RS. Fungal endophytes: diversity and functional roles. New Phytol. 2009;182:314–30.
Usuki F, Narisawa K. A mutualistic symbiosis between a dark septate endophytic fungus, Heteroconium chaetospira, and a nonmycorrhizal plant, Chinese cabbage. Mycologia. 2007;99:175–84.
Yadav V, Kumar M, Deep DK, Kumar H, Sharma R, Tripathi T, et al. A phosphate transporter from the root endophytic fungus Piriformospora indica plays a role in phosphate transport to the host plant. J Biol Chem. 2010;285:26532–44.
Busby PE, Ridout M, Newcombe G. Fungal endophytes: modifiers of plant disease. Plant Mol Biol. 2016;90:645–55.
Clay K. Interactions among fungal endophytes, grasses and herbivores. Res Popul Ecol. 1996;38:191–201.
Omacini M, Chaneton EJ, Ghersa CM, Müller CB. Symbiotic fungal endophytes control insect host-parasite interaction webs. Nature. 2001;409:78–81.
Bultman TL, Bell GD. Interaction between fungal endophytes and environmental stressors influences plant resistance to insects. Oikos. 2003;103:182–90.
Rodriguez RJ, Henson J, Volkenburgh EV, Hoy M, Wright L, Beckwith F, et al. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008;2:404.
Gill SS, Gill R, Trivedi DK, Anjum NA, Sharma KK, Ansari MW, et al. Piriformospora indica: potential and Significance in plant stress tolerance. Front Microbiol. 2016;7:1–20.
Clay K, Holah J. Fungal endophyte symbiosis and plant diversity in successional fields. Science. 1999;285:1742–4.
Afkhami ME, Strauss SY. Native fungal endophytes suppress an exotic dominant and increase plant diversity over small and large spatial scales. Ecology. 2016;97:1159–69.
Uchitel A, Omacini M, Chaneton EJ. Inherited fungal symbionts enhance establishment of an invasive annual grass across successional habitats. Oecologia. 2011;165:465–75.
Aschehoug ET, Metlen KL, Callaway RM, Newcombe G. Fungal endophytes directly increase the competitive effects of an invasive forb. Ecology. 2012;93:3–8.
Dickie IA, Bufford JL, Cobb RC, Desprez‐Loustau M-L, Grelet G, Hulme PE, et al. The emerging science of linked plant–fungal invasions. New Phytol. 2017;215:1314–32.
Kowalski KP, Bacon C, Bickford W, Braun H, Clay K, Leduc-Lapierre M, et al. Advancing the science of microbial symbiosis to support invasive species management: a case study on Phragmites in the Great Lakes. Front Microbiol. 2015;6:1–14.
Martin LJ, Blossey B. The runaway weed: costs and failures of phragmites australis management in the USA. Estuaries Coasts. 2013;36:626–32.
Hazelton ELG, Mozdzer TJ, Burdick DM, Kettenring KM, Whigham DF. Phragmites australis management in the United States: 40 years of methods and outcomes. AoB Plants. 2014;6:1–19.
Farnsworth EJ, Meyerson LA. Species composition and inter-annual dynamics of a freshwater tidal plant community following removal of the invasive grass, Phragmites Australis. Biol Invasions. 1999;1:115–27.
Meyerson LA, Saltonstall K, Windham L, Kiviat E, Findlay S. A comparison of Phragmites Australisin freshwater and brackish marsh environments in North America. Wetl Ecol Manag. 2000;8:89–103.
Silliman BR, Bertness MD. Shoreline development drives invasion of Phragmites Australis and the loss of plant diversity on New England salt marshes. Conserv Biol. 2004;18:1424–34.
Rooth JE, Stevenson JC. Sediment deposition patterns in Phragmites australiscommunities: implications for coastal areas threatened by rising sea-level. Wetl Ecol Manag. 2000;8:173–83.
Mozdzer TJ, Megonigal JP. Increased methane emissions by an introduced Phragmites Australis lineage under global change. Wetlands. 2013;33:609–15.
Bernal B, Megonigal JP, Mozdzer TJ. An invasive wetland grass primes deep soil carbon pools. Glob Change Biol. 2017;23:2104–16.
Rice D, Rooth J, Stevenson JC. Colonization and expansion of Phragmites australis in upper Chesapeake Bay tidal marshes. Wetlands. 2000;20:280.
Packett CR, Chambers RM. Distribution and nutrient status of haplotypes of the marsh grass Phragmites australis along the Rappahannock River in Virginia. Estuaries Coasts. 2006;29:1222–5.
Able KW, Hagan SM, Brown SA. Mechanisms of marsh habitat alteration due toPhragmites: response of young-of-the-year mummichog (Fundulus heteroclitus) to treatment for Phragmites removal. Estuaries. 2003;26:484–94.
Saltonstall K. Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc Natl Acad Sci USA. 2002;99:2445–9.
Vasquez EA, Glenn EP, Brown JJ, Guntenspergen GR, Nelson SG. Salt tolerance underlies the cryptic invasion of North American salt marshes by an introduced haplotype of the common reed Phragmites australis (Poaceae). Mar Ecol Prog Ser. 2005;298:1–8.
Meadows RE, Saltonstall K. Distribution of native and introduced Phragmites australis in freshwater and oligohaline tidal marshes of the Delmarva peninsula and southern New Jersey1. tbot. 2007;134:99–107.
Glenn EP. Relationship between cation accumulation and water content of salt‐tolerant grasses and a sedge. Plant Cell Environ. 1987;10:205–12.
Lissner J, Schierup H-H. Effects of salinity on the growth of Phragmites australis. Aquat Bot. 1997;55:247–60.
Amsberry L, Baker MA, Ewanchuk PJ, Bertness MD. Clonal integration and the expansion of Phragmites Australis. Ecol Appl. 2000;10:1110–8.
Bart D, Hartman JM. The role of large rhizome dispersal and low salinity windows in the establishment of common reed, phragmites australis, in salt marshes: new links to human activities. Estuaries. 2003;26:436–43.
Soares MA, Li H-Y, Kowalski KP, Bergen M, Torres MS, White JF. Evaluation of the functional roles of fungal endophytes of Phragmites australis from high saline and low saline habitats. Biol Invasions. 2016;18:2689–702.
Weishampel PA, Bedford BL. Wetland dicots and monocots differ in colonization by arbuscular mycorrhizal fungi and dark septate endophytes. Mycorrhiza. 2006;16:495–502.
Kandalepas D, Stevens KJ, Shaffer GP, Platt WJ. How abundant are root-colonizing fungi in Southeastern Louisiana’s degraded marshes? Wetlands. 2010;30:189–99.
Jumpponen A, Trappe JM. Dark septate endophytes: a review of facultative biotrophic root‐colonizing fungi. New Phytol. 1998;140:295–310.
Mandyam K, Jumpponen A. Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud Mycol. 2005;53:173–89.
Read DJ, Wandter KH. Observations on the mycorrhizal status of some alpine plant communities. New Phytol. 1981;88:341–52.
Barrow J, Aaltonen R. Evaluation of the internal colonization of Atriplex canescens (Pursh) Nutt. roots by dark septate fungi and the influence of host physiological activity. Mycorrhiza. 2001;11:199–205.
Rains KC, Nadkarni NM, Bledsoe CS. Epiphytic and terrestrial mycorrhizas in a lower montane Costa Rican cloud forest. Mycorrhiza. 2003;13:257–64.
Wang J, Li T, Liu G, Smith JM, Zhao Z. Unraveling the role of dark septate endophyte (DSE) colonizing maize (Zea mays) under cadmium stress: physiological, cytological and genic aspects. Sci Rep. 2016;6:1–12.
Li X, He X, Hou L, Ren Y, Wang S, Su F. Dark septate endophytes isolated from a xerophyte plant promote the growth of Ammopiptanthus mongolicus under drought condition. Sci Rep. 2018;8:1–11.
Zhu L, Li T, Wang C, Zhang X, Xu L, Xu R, et al. The effects of dark septate endophyte (DSE) inoculation on tomato seedlings under Zn and Cd stress. Environ Sci Pollut Res. 2018;25:35232–41.
Hill PW, Broughton R, Bougoure J, Havelange W, Newsham KK, Grant H, et al. Angiosperm symbioses with non-mycorrhizal fungal partners enhance N acquisition from ancient organic matter in a warming maritime Antarctic. Ecol Lett. 2019;22:2111–9.
U.S. Fish and Wildlife Service., Cowardin L. Classification of wetlands and deepwater habitats of the United States. Washington D.C.: Fish and Wildlife Service Office of Biological Services U.S. Dept. of the Interior; 1995.
Holdredge C, Bertness MD, Wettberg EV, Silliman BR. Nutrient enrichment enhances hidden differences in phenotype to drive a cryptic plant invasion. Oikos. 2010;119:1776–84.
Ban Y, Tang M, Chen H, Xu Z, Zhang H, Yang Y. The response of dark septate endophytes (DSE) to heavy metals in pure culture. PLoS ONE. 2012;7:1–11.
Burke DJ. Effects of Alliaria petiolata (garlic mustard; Brassicaceae) on mycorrhizal colonization and community structure in three herbaceous plants in a mixed deciduous forest. Am J Bot. 2008;95:1416–25.
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990;115:495–501.
R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. https://www.R-project.org/.
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–3.
Nilsson R. The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019;47:D259–64.
McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8:e61217.
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: community ecology Package. 2019. https://CRAN.R-project.org/package=vegan.
Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag; 2016. http://ggplot2.org.
Hervé M. RVAideMemoire: testing and plotting procedures for biostatistics. 2019. https://CRAN.R-project.org/package=RVAideMemoire.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Wang Y, Naumann U, Eddelbubettel D, Wilshire J. mvabund: statistical methods for analysing multivariate abundance data. Methods in Ecol Evol. 2012;3:471–4.
Lenth R. emmeans: estimated marginal means, aka least-squares means. 2019. https://CRAN.R-project.org/package=emmeans.
Xie L, Chen Y-L, Long Y-Y, Zhang Y, Liao S-T, Liu B, et al. Three new species of Conlarium from sugarcane rhizosphere in southern China. MycoKeys. 2019;56:1–11.
Vohník M, Borovec O, Župan Ivan, Kolařík M, Sudová R. Fungal root symbionts of the seagrass Posidonia oceanica in the central Adriatic Sea revealed by microscopy, culturing and 454-pyrosequencing. Mar Ecol Prog Ser. 2017;583:107–20.
Redman RS, Kim YO, Woodward CJDA, Greer C, Espino L, Doty SL, et al. Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: a strategy for mitigating impacts of climate change. PLOS ONE. 2011;6:e14823.
Mandyam KG, Jumpponen A. Mutualism–parasitism paradigm synthesized from results of root-endophyte models. Front Microbiol. 2015;5:1–15.
Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, et al. A meta‐analysis of context‐dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett. 2010;13:394–407.
Newsham KK. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011;190:783–93.
Mayerhofer MS, Kernaghan G, Harper KA. The effects of fungal root endophytes on plant growth: a meta-analysis. Mycorrhiza. 2013;23:119–28.
Ban Y, Xu Z, Yang Y, Zhang H, Chen H, Tang M. Effect of dark septate endophytic fungus gaeumannomyces cylindrosporus on plant growth, photosynthesis and Pb tolerance of maize (Zea mays L.). Pedosphere. 2017;27:283–92.
He Y, Yang Z, Li M, Jiang M, Zhan F, Zu Y, et al. Effects of a dark septate endophyte (DSE) on growth, cadmium content, and physiology in maize under cadmium stress. Environ Sci Pollut Res. 2017;24:18494–504.
Qin Y, Druzhinina IS, Pan X, Yuan Z. Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol Adv. 2016;34:1245–59.
Zhan F, He Y, Zu Y, Li T, Zhao Z. Characterization of melanin isolated from a dark septate endophyte (DSE), Exophiala pisciphila. World J Microbiol Biotechnol. 2011;27:2483–9.
Santander C, Aroca R, Ruiz-Lozano JM, Olave J, Cartes P, Borie F, et al. Arbuscular mycorrhiza effects on plant performance under osmotic stress. Mycorrhiza. 2017;27:639–57.
Kothari SK, Marschner H, George E. Effect of VA mycorrhizal fungi and rhizosphere microorganisms on root and shoot morphology, growth and water relations in maize. New Phytol. 1990;116:303–11.
Neumann, E. George, E. Nutrient Uptake: The Arbuscular Mycorrhiza Fungal Symbiosis as a Plant Nutrient Acquisition Strategy. In: Koltai H and Kapulnik Y (eds). Arbuscular Mycorrhizas: Physiology and Function. 2nd edn. (Springer Science+Business Media B.V., 2010) pp 137–67.
Yarwood SA, Baldwin AH, Mateu MG, Buyer JS. Archaeal rhizosphere communities differ between the native and invasive lineages of the wetland plant Phragmites australis (common reed) in a Chesapeake Bay subestuary. Biol Invasions. 2016;18:2717–28.
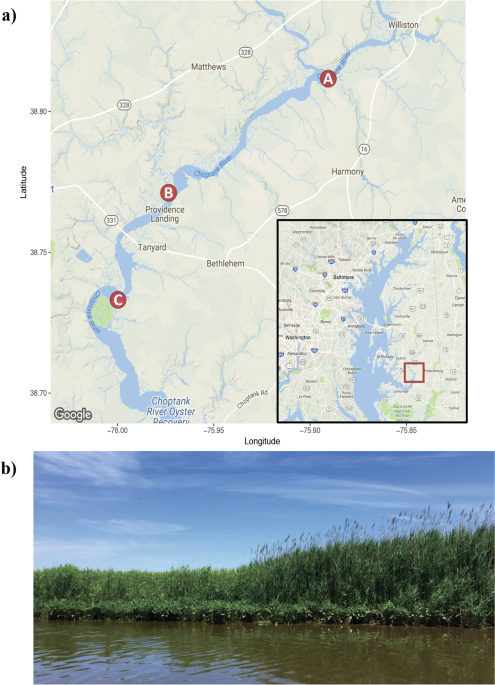
Dorfman DS, Mabrouk A, Bauer L, Nelson DM, Clement C, Claflin L. Choptank Ecological Assessment: Digital Atlas: Baseline Status Report. NOAA Technical Memorandum NOS NCCOS 213. 2016. Silver Spring, MD. pp 173.
Emery NC, Ewanchuk PJ, Bertness MD. Competition and salt-marsh plant zonation: stress tolerators may be dominant competitors. Ecology. 2001;82:2471–85.
Maurer DA, Zedler JB. Differential invasion of a wetland grass explained by tests of nutrients and light availability on establishment and clonal growth. Oecologia. 2002;131:279–88.
Bickford WA, Goldberg DE, Kowalski KP, Zak DR. Root endophytes and invasiveness: no difference between native and non-native Phragmites in the Great Lakes Region. Ecosphere. 2018;9:1–14.
Xie L, Chen Y-L, Long Y-Y, Zhang Y, Liao S-T, Liu B, et al. Three new species of Conlarium from sugarcane rhizosphere in southern China. MycoKeys. 2019;56:1–11.
Kandalepas D, Blum MJ, Van Bael SA. Shifts in symbiotic endophyte communities of a foundational salt marsh grass following oil exposure from the deepwater horizon oil spill. PLoS ONE. 2015;10:1–18.
Ruotsalainen A, Väre H, Vestberg M. Seasonality of root fungal colonization in low-alpine herbs. Mycorrhiza. 2002;12:29–36.
Mandyam K, Jumpponen A. Seasonal and temporal dynamics of arbuscular mycorrhizal and dark septate endophytic fungi in a tallgrass prairie ecosystem are minimally affected by nitrogen enrichment. Mycorrhiza. 2008;18:145–55.
Herbert ER, Boon P, Burgin AJ, Neubauer SC, Franklin RB, Ardón M, et al. A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere. 2015;6:art206.
Tully KL, Weissman D, Wyner WJ, Miller J, Jordan T. Soils in transition: saltwater intrusion alters soil chemistry in agricultural fields. Biogeochemistry. 2019;142:339–56.
Koch MS, Mendelssohn IA, McKee KL. Mechanism for the hydrogen sulfide-induced growth limitation in wetland macrophytes. Limnology and Oceanography. 1990;35:399–408.
Seliskar DM, Smart KE, Higashikubo BT, Gallagher JL. Seedling sulfide sensitivity among plant species colonizing Phragmites-infested wetlands. Wetlands. 2004;24:426–33.
Source: Ecology - nature.com


