Moore, J., Adamo, S. & Thomas, F. Manipulation: expansion of the paradigm. Behav. Processes 68, 283–287 (2005).
Dawkins, R. The extended phenotype: the long reach of the gene. Oxford University Press, Oxford (1982).
Andersen, S. B. et al. The life of a dead ant: the expression of an adaptive extended phenotype. Am. Nat. 174, 424–433 (2009).
Hughes, D. P. et al. Behavioral mechanisms and morphological symptoms of zombie ants dying from fungal infection. BMC Ecol. 11, 13 (2011).
Berger, V., Galaktionov, K. V. & Prokof’ev, V. V. Effect of parasites on host adaptation to abiotic environmental factors: host-parasite relationship of trematode parthenites–mollusc system. Parazitologiia 35, 192–200 (2001).
Poulin, R. Evolutionary ecology of parasites. Princeton University Press, Princeton (2011).
Schmid-Hempel, P. Evolutionary parasitology the integrated study of infections, immunology, ecology, and genetics. Oxford University Press, Oxford (2011)
Combes, C. Parasitism: the ecology and evolution of intimate interactions. University of Chicago Press, Chicago (2001)
Evans, H. C., Elliot, S. L. & Hughes, D. P. Ophiocordyceps unilateralis: A keystone species for unraveling ecosystem functioning and biodiversity of fungi in tropical forests? Commun. Integr. Biol. 4, 598–602 (2011).
Pontoppidan, M. B., Himaman, W., Hywel-Jones, N. L., Boomsma, J. J. & Hughes, D. P. Graveyards on the move: the spatio-temporal distribution of dead ophiocordyceps-infected ants. Plos One 4, e4835 (2009).
Evans, H. C., Elliot, S. L. & Hughes, D. P. Hidden diversity behind the zombie-ant fungus Ophiocordyceps unilateralis: four new species described from carpenter ants in Minas Gerais, Brazil. Plos One 6, e17024 (2011).
Luangsa-Ard, J. J., Ridkaew, R., Tasanathai, K., Thanakitpipattana, D. & Hywel-Jones, N. Ophiocordyceps halabalaensis: a new species of Ophiocordyceps pathogenic to Camponotus gigas in Hala Bala Wildlife Sanctuary, Southern Thailand. Fungal. Biol. 115, 608–614 (2011).
Kepler, R. M., Kaitsu, Y., Tanaka, E., Shimano, S. & Spatafora, J. W. Ophiocordyceps pulvinata sp. nov., a pathogen of ants with a reduced stroma. Mycoscience 52, 39–47 (2011).
Kobmoo, N., Mongkolsamrit, S., Tasanathai, K., Thanakitpipattana, D. & Luangsa-Ard, J. J. Molecular phylogenies reveal host-specific divergence of Ophiocordyceps unilateralis sensu lato following its host ants. Mol. Ecol. 21, 3022–3031 (2012).
Araújo, J. P. M., Evans, H. C., Geiser, D. M., Mackay, W. P. & Hughes, D. P. Unravelling the diversity behind the Ophiocordyceps unilateralis (Ophiocordycipitaceae) complex: Three new species of zombie-ant fungi from the Brazilian Amazon. Phytotaxa 220, 224–238 (2015).
Chang, L.-W. et al. Species composition, size-class structure and diversity of the Lienhuachih forest dynamics plot in a subtropical evergreen broad-leaved forest in central Taiwan. Taiwan J. For. Sci. 25, 81–95 (2010).
Sun, P.-F., Chen, P.-H. & Lin, W.-J. Lin, C.-C. & Chou, J.-Y. Variation in the ability of fungi in the extrafloral nectar of Mallotus paniculatus to attract ants as plant defenders. Mycosphere 9, 178–188 (2018).
Rehner, S. A. & Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97, 84–98 (2005).
Glass, N. L. & Donaldson, G. C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 61, 1323–1330 (1995).
Edgar, R. C. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5, 113 (2004).
Hall, T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Window 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98 (1999).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
Felsenstein, J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791 (1985).
Clement, M., Posada, D. & Crandall, K. A. TCS: a computer program to estimate gene genealogies. Mol. Ecol. 9, 1657–1659 (2000).
Templeton, A. R., Crandall, K. A. & Sing, C. F. A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genetics 132, 619–633 (1992).
Nguyen, L. T., Schmidt, H. A., Von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015).
Puillandre, N., Lambert, A., Brouillet, S. & Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 21, 1864–1877 (2012).
Lin, C.-C. & Wu, W.-J. The ant fauna of Taiwan (Hymenoptera: Formicidae), with the keys to subfamilies and genera. Annu. Natl. Taiwan Mus. 46, 5–69 (2003).
Terayama, M. A synopsis of the family Formicidae of Taiwan (Insecta: Hymenoptera). Liberal Arts, Bull. Kanto Gakuen Univ. 17, 81–266 (2009).
Leong, C.-M., Hsiao, Y. & Shiao, S.-F. Polyrhachis (Cyrtomyrma) debilis Emery, 1887 (Hymenoptera: Formicidae), a New Record of Ant Species in Taiwan. Formosan Entomologist 35, 143–147 (2015).
Chiotis, M., Jermiin, L. S. & Crozier, R. H. A molecular framework for the phylogeny of the ant subfamily Dolichoderinae. Mol. Phylogenet. Evol. 17, 108–116 (2000).
Yeung, E. C. The use of histology in the study of plant tissue culture systems—some practical comments. In Vitro Cell. Dev. 35, 137–143 (1999).
Zhang, N. et al. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microbiol. 44, 2186–2190 (2006).
Pal, M., Dave, P. & Manna, A. K. Emerging role of Aspergillus flavus in human and animal disorders. J. Mycopathol. Res. 52, 211–216 (2014).
Srinivas, C. et al. Fusarium oxysporum f. sp. lycopersici causal agent of vascular wilt disease of tomato: Biology to diversity–A review. Saudi J. Biol. Sci. 26, 1315–1324 (2019).
Futuyma, D. J. & Moreno, G. The evolution of ecological specialization. Annu. Rev. Ecol. Evol. Syst. 19, 207–233 (1988).
Agosta, S. J., Janz, N. & Brooks, D. R. How specialists can be generalists: resolving the” parasite paradox” and implications for emerging infectious disease. Zoologia (Curitiba) 27, 151–162 (2010).
Fallon, S. M., Bermingham, E. & Ricklefs, R. E. Host specialization and geographic localization of avian malaria parasites: a regional analysis in the Lesser Antilles. Am. Nat. 165, 466–480 (2005).
Combes, C. Ethological aspects of parasite transmission. Am. Nat. 138, 866–880 (1991).
Bush, A. O. & Kennedy, C. R. Host fragmentation and helminth parasites: hedging your bets against extinction. Int. J. Parasitol. 24, 1333–1343 (1994).
Cooper, N. et al. Phylogenetic host specificity and understanding parasite sharing in primates. Ecol. Lett. 15, 1370–1377 (2012).
Burns, J. H. & Strauss, S. Y. More closely related species are more ecologically similar in an experimental test. Proc. Natl. Acad. Sci. USA 108, 5302–5307 (2011).
Huang, S., Bininda-Emonds, O. R., Stephens, P. R., Gittleman, J. L. & Altizer, S. Phylogenetically related and ecologically similar carnivores harbour similar parasite assemblages. J. Anim. Ecol. 83, 671–680 (2014).
Nosil, P. & Mooers, A. Testing hypotheses about ecological specialization using phylogenetic trees. Evolution 59, 2256–2263 (2005).
Janz, N., Nyblom, K. & Nylin, S. Evolutionary dynamics of host-plant specialization: a case study of the tribe Nymphalini. Evolution 55, 783–796 (2001).
West-Eberhard, M. J. Developmental plasticity and evolution. Oxford University Press, Oxford (2003).
Lande, R. & Arnold, S. J. The measurement of selection on correlated characters. Evolution 37, 1210–1226 (1983).
Gould, S. J. & Lewontin, R. C. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc. R. Soc. B 205, 581–598 (1979).
Janzen, D. H. & Martin, P. S. Neotropical anachronisms: the fruits the gomphotheres ate. Science 215, 19–27 (1982).
McLennan, D. A. & Brooks, D. R. Complex histories of speciation and dispersal in communities: a re-analysis of some Australian bird data using BPA. J. Biogeogr. 29, 1055–1066 (2002).
Janz, N., Nylin, S. & Wahlberg, N. Diversity begets diversity: host expansions and the diversification of plant-feeding insects. BMC Evol. Biol. 6, 4 (2006).
Janz, N. & Nylin, S. The oscillation hypothesis of host-plant range and speciation. In: Tilmon, K. (ed.) Specialization, speciation, and radiation: the evolutionary biology of herbivorous insects. University of California Press, Oakland, pp 203–215 (2008).
Nylin, S. & Janz, N. Butterfly host plant range: an example of plasticity as a promoter of speciation? Evol. Ecol. 23, 137–146 (2009).
Mayr, E. Animal species and evolution. Harvard University Press, Cambridge (1963).
Thompson, J. N. The coevolutionary process. University of Chicago Press, Chicago (1994).
Bush, G. L. Sympatric speciation in animals: new wine in old bottles. Trends Ecol. Evol. 9, 285–288 (1994).
Berlocher, S. H. & Feder, J. L. Sympatric speciation in phytophagous insects: moving beyond controversy? Annu. Rev. Entomol. 47, 773–815 (2002).
Agosta, S. J. & Klemens, J. A. Ecological fitting by phenotypically flexible genotypes: implications for species associations, community assembly and evolution. Ecol. Lett. 11, 1123–1134 (2008).
Robson, S. K. & Kohout, R. J. A review of the nesting habits and socioecology of the ant genus Polyrhachis Fr. Smith. Asian Myrmecol. 1, 81–99 (2007).
Severinghaus, L. L. Cavity dynamics and breeding success of the Lanyu Scops Owl (Otus elegans). J. Ornithol. 148, 407–416 (2007).
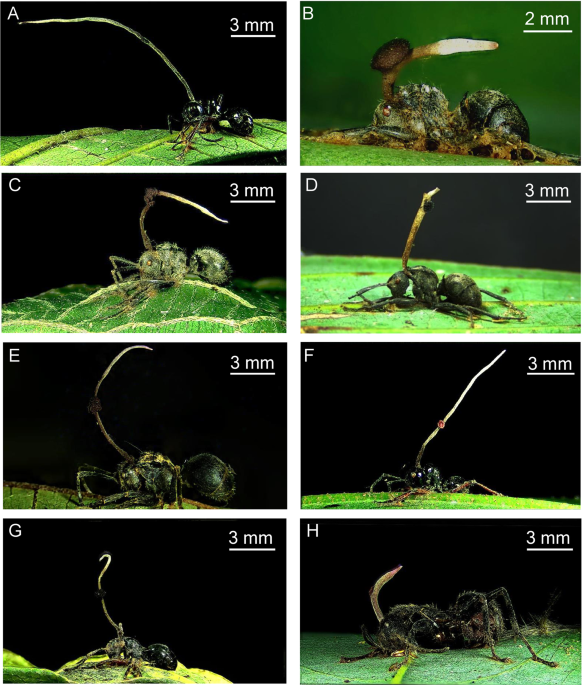
Mongkolsamrit, S. et al. Life cycle, host range and temporal variation of Ophiocordyceps unilateralis/Hirsutella formicarum on Formicine ants. J. Invertebr. Pathol. 111, 217–224 (2012).
Hughes, D. P., Wappler, T. & Labandeira, C. C. Ancient death-grip leaf scars reveal ant-fungal parasitism. Biol. Lett. 7, 67–70 (2011).
Loreto, R. G., Elliot, S. L., Freitas, M. L., Pereira, T. M. & Hughes, D. P. Long-term disease dynamics for a specialized parasite of ant societies: a field study. Plos One 9, e103516 (2014).
Kepler, R. M., Kaitsu, Y., Tanaka, E., Shimano, S. & Spatafora, J. W. Ophiocordyceps pulvinata sp. nov., a pathogen with a reduced stroma. Mycoscience 52, 39–47 (2011).
De Bekker, C. et al. Species-specific ant brain manipulation by a specialized fungal parasite. BMC Evol. Biol. 14, 166 (2014).
Loreto, R. G. et al. Evidence for convergent evolution of host parasitic manipulation in response to environmental conditions. Evolution 72, 2144–2155 (2018).
Vega, F. E. Insect pathology and fungal endophytes. J. Invertebr. Pathol. 98, 277–279 (2008).
Vidal, S. & Jaber, L. R. Entomopathogenic fungi as endophytes: plant–endophyte–herbivore interactions and prospects for use in biological control. Curr. Sci. 109, 46–54 (2015).
Chung, T.-Y. et al. Zombie ant heads are oriented relative to solar cues. Fungal. Ecol. 25, 22–28 (2017).
Source: Ecology - nature.com


