Farrell, B. D. et al. The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution. 55, 2011–2027 (2001).
Hulcr, J. & Stelinski, L. L. The ambrosia symbiosis: from evolutionary ecology to practical management. Annu. Rev. Entomol. 62, 285–303 (2017).
Kirkendall, L. R. The evolution of mating systems in bark and ambrosia beetles (Coleoptera: Scolytineae and Platypodidae). Zool. J. Linn. Soc. 77, 293–352 (1983).
Fraedrich, S. W. et al. A Fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other lauraceae in the southeastern United States. Plant Dis. 92, 215–224 (2008).
Paine, T. D., Raffa, K. F. & Harrington, T. C. Interactions among Scolytine bark beetles, their associated fungi, and live host conifers. Annu. Rev. Entomol. 42, 179–206 (1997).
Batra, L. R. Ambrosia fungi: extent of specificity to ambrosia beetles. Science. 153, 193–195 (1966).
Carrillo, D. et al. Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant Pathol. 63, 54–62 (2014).
Kostovcik, M. et al. The ambrosia symbiosis is specific in some species and promiscuous in others: evidence from community pyrosequencing. ISME J. 9, 126–138 (2015).
Saucedo-Carabez, J. R., Ploetz, R. C., Konkol, J. L., Carrillo, D. & Gazis, R. Partnerships between ambrosia beetles and fungi: lineage-specific promiscuity among vectors of the laurel wilt pathogen, Raffaelea lauricola. Microb. Ecol. 76, 925–940 (2018).
Wingfield, M. J. et al. Novel associations between ophiostomatoid fungi, insects and tree hosts: current status—future prospects. Biol. Invasions. 19, 3215–3228 (2017).
Rabaglia, R. J., Dole, S. A. & Cognato, A. I. Review of American Xyleborina (Coleoptera; Curculionidae: Scolytinae) occuring north of Mexico, with an illustrated key. Ann. Entomol. Soc. Am. 99, 1034–1056 (2006).
Kendra, P. E., Montgomery, W. S., Niogret, J. & Epsky, N. D. An uncertain future for American Lauraceae: A lethal threat from redbay ambrosia beetle and laurel wilt disease (A Review). Am. J. Plant Sci. 4, 727–738 (2013).
Mayfield, A. et al. Suitability of California bay laurel and other species as hosts for the non-native redbay ambrosia beetle and granulate ambrosia beetle. Agric. For. Entomol. 15, 227–235 (2013).
(USDA NASS) USDA National Agricultural Statistics Survey Quick Stats. https://quickstats.nass.usda.gov/results/594CE3F3-9DCB-3D62-983D-5485A8CD27B3?pivot=short_desc).15 December 2018 (2018).
Evans, E. A., Crane, J., Hodges, A. & Osborne, J. L. Potential economic impact of laurel wilt disease on the Florida avocado industry. HortTechnology. 20, 234–238 (2010).
Ploetz, R. C. et al. Laurel Wilt, Caused by Raffaelea lauricola, is Confirmed in Miami-Dade County, Center of Florida’s Commercial Avocado Production. Plant Dis. 95, 1589–1589 (2011).
Crane, J. H., Balerdi, C. & Maguire, I. Avocado Growing in the Florida Landscape, Gainesville, FL, USA: Institute of Food and Agricultural Sciences Extension, University of Florida Circular 1034. [http://edis.ifas.ufl.edu/mg213] Accessed March 23, 2019 (2007).
Knight, R. L. History, distribution, and uses. In: Whiley, A. W., Shaffer, B. & Wholstenholm, N. eds. The Avocado: Botany, Production, and Uses (2002).
Ploetz, R. C. et al. Responses of avocado to laurel wilt, caused by Raffaelea lauricola. Plant Pathol. 61, 801–808 (2012).
Mayfield, A. E. et al. Ability of the redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae) to bore into young avocado (Lauraceae) plants and transmit the laurel wilt pathogen (Raffaelea sp). Fla. Entomol. 91, 485–487 (2008).
Kendra, P. E. et al. Evaluation of seven essential oils identifies cubeb oil as most effective attractant for detection of Xyleborus glabratus. J. Pest Sci. 87, 681–689 (2014).
Carrillo, D., Duncan, R. E. & Peña, J. E. Ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) that breed in avocado wood in Florida. Fla. Entomol. 95, 573–579 (2012).
Menocal, O., Kendra, P. E., Montgomery, W. S., Crane, J. H. & Carrillo, D. Vertical distribution and daily flight periodicity of ambrosia beetles (Coleoptera: Curculionidae) in Florida avocado orchards affected by laurel wilt. J. Econ. Entomol. 111, 1190–1196 (2018).
Hulcr, J., Mann, R. & Stelinski, L. L. The scent of a partner: ambrosia beetles are attracted to volatiles from their fungal symbionts. J. Chem. Ecol. 37, 1374–1377 (2011).
Cognato, A. I., Hulcr, J., Dole, S. A. & Jordal, B. H. Phylogeny of haplo-diploid, fungus-growing ambrosia beetles (Curculionidae: Scolytinae: Xyleborini) inferred from molecular and morphological data. Zool. Scr. 40, 174–186 (2011).
Saucedo, J. R. et al. Nutritional symbionts of a putative vector, Xyleborus bispinatus, of the laurel wilt pathogen of avocado, Raffaelea lauricola. Symbiosis. 75, 29–38 (2018).
Hughes, M. A. et al. Evaluation of repellents for the redbay ambrosia beetle, Xyleborus glabratus, vector of the laurel wilt pathogen. J. Appl. Entomol. 141, 653–664 (2017).
Kendra, P. E. et al. North American Lauraceae: terpenoid emissions, relative attraction and boring preferences of redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae). Plos One 9, e102086 (2014).
Ranger, C. M., Reding, M. E., Persad, A. B. & Herms, D. A. Ability of stress-related volatiles to attract and induce attacks by Xylosandrus germanus and other ambrosia beetles. Agric. For. Entomol. 12, 177–185 (2010).
Byers, A. B., Sadowsky, A. & Levi-Zada, A. Index of host habitat preference explored by movement-based simulations and trap captures. J Anim Ecol. 87, 1320–1330 (2018).
Gomez, D. F. et al. Species delineation within the Euwallacea fornicates (Coleoptera: Curculionidae) complex revealed by morphometric and phylogenetic analyses. Insect Syst. Divers. 2(6), 1–11 (2018).
Menocal, O. et al. Rearing Xyleborus volvulus (Coleoptera: Curculionidae) on media containing sawdust from avocado or silkbay, with or without Raffaelea lauricola (Ophiostomatales: Ophiostomataceae). Environ. Entomol. 46, 1275–1283 (2017).
Menocal, O. et al. Xyleborus bispinatus reared on artificial media in the presence or absence of the laurel wilt pathogen (Raffaelea lauricola). Insects. 9 (2018).
Martini, X. et al. The fungus Raffaelea lauricola modified behavior of its symbiont and vector, the redbay ambrosia beetle (Xyleborus Glabratus), by altering host plant volatile production. J Chem Ecol 43, 519–51 (2017).
Martini, X., Hughes, M. A., Smith, J. A. & Stelinski, L. L. Attraction of redbay ambrosia beetle, Xyleborus glabratus, to leaf volatiles of its host plants in North America. J. Chem. Ecol. 41, 613–621 (2015).
Hughes, M. A. et al. Recovery plan for laurel wilt on redbay and other forest species caused by Raffaelea lauricola and disseminated by xyleborus glabratus (2015).
Rodgers, L., Derksen, A. & Pernas, T. Expansion and impact of laurel wilt in the Florida Everglades. Florida Entomol. 97, 1247–1250 (2014).
Ploetz, R. C. et al. Recovery Plan for Laurel Wilt of Avocado, caused by Raffaelea lauricola. Plant Health Progress. 18, 51–77, https://doi.org/10.1094/PHP-12-16-0070-RP (2017).
Cook, S. M., Khan, Z. R. & Pickett, J. A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 52, 375–400 (2007).
Bentz, A. B. J., Kegley, S., Gibson, K. & Thier, R. A test of high-dose Verbenone for stand-level protection of lodgepole and whitebark pine from mountain pine beetle (Coleoptera: Curculionidae: Scolytinae) attacks. 98, 1614–1621 (2005).
Borden, J. H., Birmingham, A. L. & Burleigh, J. S. Evaluation of the push-pull tactic against the mountain pine beetle using verbenone and non-host volatiles in combination with pheromone-baited trees. For. Chron. 82, 579–590 (2006).
Gillette, N. E. et al. Verbenone-releasing flakes protect individual Pinus contorta trees from attack by Dendroctonus ponderosae and Dendroctonus valens (Coleoptera: Curculionidae, Scolytinae). Agric. For. Entomol. 8, 243–251 (2006).
Burbano, E. G. et al. Efficacy of traps, lures, and repellents for Xylosandrus compactus (Coleoptera: Curculionidae) and other ambrosia beetles on Coffea arabica plantations and Acacia koa nurseries in Hawaii. Environ. Entomol. 41, 133–140 (2012).
Fettig, C. Efficacy of SPLAT Verb for protecting individual Pinus contorta, Pinus ponderosa, and Pinus lambertiana from mortality attributed to dendroctonus ponderosae. CBC News. 113, 11–20 (2017).
Holopainen, J. K. & Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 15, 176–184 (2010).
Byers, J. A., Anderbrant, O. & Löqvist, J. Effective attraction radius. J. Chem. Ecol. 15, 749–765 (1989).
Seo, M., Martini, X., Rivera, M. J. & Stelinski, L. L. Flight Capacities and Diurnal flight patterns of the ambrosia beetles, Xyleborus glabratus and Monarthrum mali (Coleoptera: Curculionidae). Environ. Entomol. 46, 729–734 (2017).
Ries, L., Fletcher, R. J., Battin, J. & Sisk, T. D. Ecological responses to habitat edges: mechanisms, models, and variability explained. Annu. Rev. Ecol. Evol. Syst. 35, 491–522 (2004).
Conlong, D. E., Webster, T. & Wilkinson, D. Ten years of area-wide integrated pest management with a push-pull component against Eldana Saccharina (Lepidoptera: Pyralidae) in sugarcane in the midlands north region of Kwazulu-Natal. Proc. S. Afr. Sug. Technol. Aes. 89, 70–84 (2016).
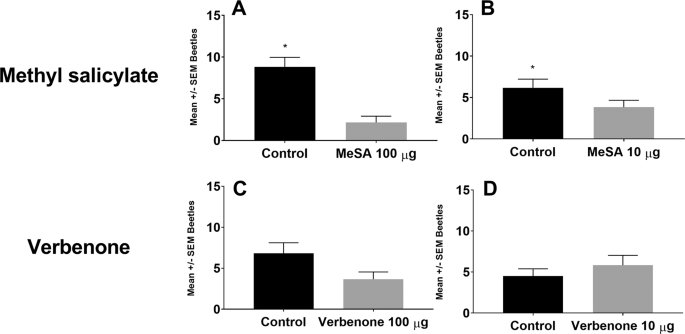
Werle, C. T. et al. Integrating repellent and attractant semiochemicals into a push-pull strategy for ambrosia beetles (Coleoptera: Curculionidae). J. Appl. Entomol. 9, 83 (2018).
Source: Ecology - nature.com


