Biotests
In all biotests, we used clonal females of Daphnia magna originating from Binnensee, a lake in North-eastern Germany (GIS coordinates 54.3256 N, 10.6296 E) inhabited by fish30, and maintained in a laboratory culture for over 20 years. The water used in the experiments (originating from the Szczęśliwice reservoir in Warsaw, Poland) had been stored in a 10 m3 underground tank for at least one month prior to the experiments. The water was aerated and filtered through a 0.45 µm filter prior to use, with microalgae Acutodesmus obliguus added as food for the Daphnia at a concentration of 1 mg of organic C/L in the life history biotest and 2 mg C/L in the vertical distribution bioassay.
Life history bioassay: effects of bile and bile salts on the life history of Daphnia
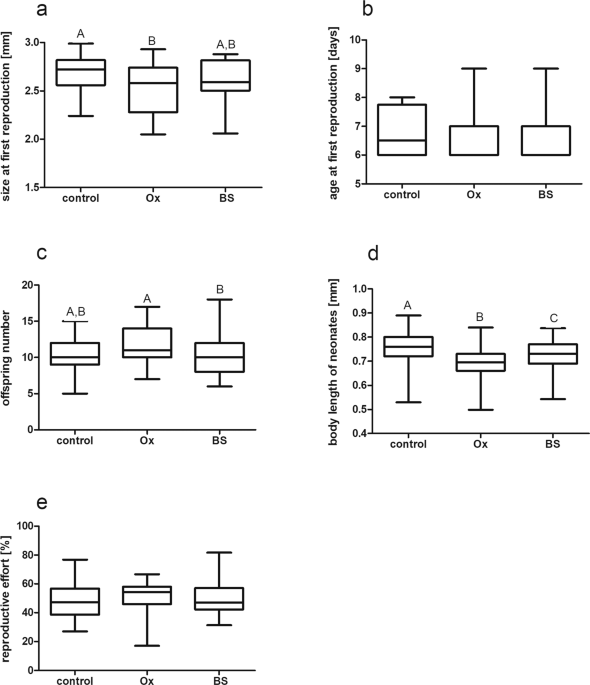
In the first experiment, we investigated the life history traits crucial for fitness of Daphnia by comparing their reaction to three treatments labeled control, ox bile – Ox, and bile salts – BS (described below). We purchased from the Sigma Aldrich company lyophilized ox bile (# 70168) and a mixture (#48305-F) of the most common primary cholic acid (CA) and its secondary form – sodium salts of deoxycholic acid (DCA), than called bile salts. Ox bile was used because we could not obtain enough Cyprinidae bile for the long term life history experiments and, moreover, it was commercially unavailable. The reagents were dissolved in distilled water to 1% stock solution and stored at −20 °C. In the bile salt (BS) and ox bile (Ox) treatments, solutions of these two substances were added to water to obtain a final concentration of 0.001%. This concentration was selected from among four potential alternatives (each applied in a gradient from 1% to 0.001% through 0.1% and 0.01%) as it caused no mortality in the Daphnia experimental population. In this pilot study, 10 neonate Daphnia were individually exposed to these concentrations of bile and bile salts until their first reproduction and the number of survivors was counted daily. Later, during the proper life history bioassays, in each treatment 10 Daphnia were cultured individually in 100 mL of medium in 250 mL capacity glass jars. Media were changed daily to maintain a sufficient amount of food and appropriate concentrations of bile and bile salts. The experiments were run at 20 °C and a 16 L:8D photoperiod and were replicated four times, i. e. 40 specimens were examined in each treatment.
In the second experiment, we investigated whether the addition of ox bile and bile salts to media containing Daphnia would reinforce the effect of an alarm substance originating from homogenized conspecifics on Daphnia traits, as fish kairomone has been found to do31. This so-called alarm substance is released from mechanically damaged Daphnia tissues and, when applied alone, acts as nonspecific cue, advertising danger from predators and initiating a series of different behavioral and life history responses in survivors32. When combined with either fish or invertebrate kairomones, it serves to advertise the type and scale of danger. In this study, the alarm substance was obtained by homogenizing 250 Daphnia individuals in 100 mL of lake water. In the alarm substance (AS) treatment, a Daphnia homogenate has been added to water in a concentration of 25 Daphnia L−1. We obtained fish kairomone (F) by keeping 20 fish (crucian carp, Carassius carassius) for 24 hours in 10 L of lake water. The resulting stock solution was then portioned and frozen, and this frozen solution was later added to the media in a concentration of 0.1 fish L−1. In the bile salt (BS + AS) and ox bile (Ox + AS) treatments, the Daphnia alarm substance was supplemented with bile and bile salts at a concentration of 0.001%. Under each treatment, 10 Daphnia were kept individually in 100 mL of medium in 250 mL capacity glass jars. Media were changed daily to maintain a sufficient amount of food and appropriate concentrations of either kairomones, alarm substances or ox bile and bile salts. The experiment was run in the incubators at 20 °C and a 16 L:8D photoperiod.
In both life history bioassays, we measured the size and age at first reproduction (the latter within 24 h accuracy), counted the number of first clutch neonates and measured their length using the NIS elements program from Nikon. Reproductive effort (ratio of neonate biomass to female biomass × 100%) was calculated for all females. Weights of individual Daphnia were calculated from the length (L)-weight (W) relationship obtained for D. magna cultured in our laboratory under the same conditions (W = 2.583 × length2.406).
Vertical distribution bioassay: effect of bile and bile salts on the diurnal depth distribution of Daphnia
The test animals were 5 day-old clonal Daphnia magna. To test the putative effects of kairomones on depth selection by Daphnia we used a device similar to that designed by Dawidowicz & Loose (1992)33 and Loose & Dawidowicz (1994)34, called a “plankton organ”, to track the diel vertical migration behavior of individual animals. It consisted of vertical glass tubes (1 m in length, 1 cm in diameter) set parallel to one another in a thermally stratified transparent water bath (20.0 °C at the top, 10.0 °C at the bottom). The setup was illuminated by halogen lamps shining overhead through a frosted glass diffuser, on a summer photoperiod (16 L:8D) setting.
In the ox-bile experiments, we tested four different media: the control medium, which was 0.45-μm filtered water from the Szczęśliwice reservoir; the fish medium (F), which was prepared by keeping a single crucian carp (7 cm total length) in 10 L of pre-filtered control water for 24 hours (equivalent to 0,1 fish L−1); the bile salts (BS) medium, which was a 0.001% solution of bile salts diluted in the pre-filtered control water; and Ox – ox bile medium, a 0.001% solution of ox-bile in the same water.
In the fish-bile experiments, we tested four different media: the control medium which was 0.45-μm filtered water from the Szczęśliwice reservoir; the fish medium (F), which was prepared by keeping a single crucian carp (7 cm total length) in 10 L of pre-filtered control water for 24 hours (equivalent to 0,1 fish L−1); and a fish bile medium, a 0.0001% v/v solution of bile extracted from the gallbladder freshly dissected from euthanized: a) the same crucian carp that was used to produce kairomone, b) common rudd Scardinius erythrophthalmus (7–8 cm) and diluted in the pre-filtered control water.
Sixty (in the ox-bile experiment) and forty five D. magna (in the fish-bile experiment) were simultaneously exposed to each of these media in 12 tubes (5 individuals per tube × 3 replicate tubes × 4 tested media and 5 individuals per tube × 3 replicate tubes × 3 tested media, respectively). Daphnia were introduced to the tubes at noon, 8 h before a simulated sunset. The next midday, 8 h after “sunrise”, the depth distribution of the animals in each tube was determined by visual counting. This procedure was repeated 3 times, each time with a new set of Daphnia exposed in freshly prepared media, giving a total of 45 depth-counts per medium treatment.
Statistical analysis
Statistical analysis was performed on the pooled results of the experimental repetitions. All results are presented as boxplots bisected at the median value with minimal and maximal whiskers. We used a non-parametric Kruskall-Wallis ANOVA to check whether the medians differed significantly between treatments and checked for differences between pairs of treatments using Dunn’s multiple comparison post-hoc test. Letters on the figures indicate statistically significant differences between the groups. Results of the Kruskall-Wallis test (H and p) as well as the p-value of Dunn’s post hoc test are presented in the tables S2–S5 in Supporting information. All calculations were made using the GraphPad Prism 5.0 program.
Chromatography
We performed chromatographic analyses of fish conditioned water, lyophilized ox bile and bile of a cyprinid fish in order to compare their bile acids/salts content.
Enrichment and extraction of fish kairomones from fish conditioned water
Twenty fish (Carassius auratus) were kept in 10 L of water for 24 hours, with no food added. The kairomone to be used for chromatographic analysis was then extracted from the water by solid-phase extraction (SPE), following a procedure initially developed and used by von Elert & Loose (1996)6. Briefly, prior to adding the sample C18-SPE cartridges (500 mg, Analytichem Int.) were preconditioned with methanol and ultrapure water (10 ml each). The pH of the sample was adjusted to 7.0 with 2 M HCl. Methanol was added to achieve a 1% concentration. The resultant solution was then passed through the cartridge and the eluate was collected. The loaded cartridge was washed with 5 ml of ultrapure water prior to elution of the isolate with 10 ml of methanol (eluent). Both the eluate and the eluent were evaporated separately until dry using a rotatory evaporator to remove organic solvents, and then resuspended in methanol. Finally, the pooled eluate + eluent was evaporated until dry and resuspended in 1 ml of methanol.
Obtaining fish bile for chromatographic analysis
Fish bile was obtained from one adult carp (Cyprinus carpio) which was purchased from a fish wholesaler. Euthanized carp was kept on ice and whole bile was aspirated directly from the gallbladder with a sterile syringe and needle. The aspirated bile (around 1 ml) was then lyophilized until dry to yield a greenish powder. This material was then dissolved in 10% methanol and used for chromatographic analyses.
All experimental protocols, methods and procedures used in the described experiments were performed in accordance with the regulations of the Polish Ethical Council for the care and use of laboratory animals, the European Community Directive for the ethical use of experimental animals, I Local Ethics Committee in Warsaw and Ordinance of minister of agriculture and rural development of December 14, 2016. In our experiment, we kept fish in the aquarium, and then fished them out, sacrificed in accordance with the guidelines approved by the I Ethics Committee in Warsaw to dissect the gallbladder.
Chromatographic analyses
The chromatographic system was previously described by Biesaga et al. 35 for polyphenols analysis. The method we used, briefly described below, has been adapted for the compounds studied in our work. Chromatographic analysis was performed using a Shimadzu LC system consisting of LC20-AD binary pumps, a DGU-20A5 degasser, a CTO-20AC column oven and a SIL-20AC autosampler connected to a 3200 QTRAP mass spectrometer (Applied Biosystem/MDS SCIEX). An electrospray ionization(ESI) was operated in negative-ion mode. ESI conditions were as follows: capillary temperature 450 °C, curtain gas at 0.3 MPa, auxiliary gas at 0.3 MPa, negative ionization mode source voltage – 4.5 kV. Nitrogen was used as curtain and auxiliary gas. For each compound, the optimum conditions of Single Reaction Mode (SRM) were determined in infusion mode (see Supporting information Table S1). A standard solution of cholic acid from an ox bile dissolved in water was infused into the electrospray source with a 50 μm i.d. PEEK capillary using a Harward Apparatus pump at 10 μL/min. Continuous mass spectra were obtained by scanning m/z from 50 to 650.
Compounds were separated on a Luna (Phenomenex) C-18 (2) column (100 × 2.1 mm, 3 µm), with the precolumn set at 30 °C. We used 8 mM of formic acid (pH 2.8) as eluent A and acetonitrile as eluent B. The mobile phase was delivered at 0.2 mL/min in gradient mode: 0–3 min. 40% B, 3–20 min 70% B, 20–25 min 70% B, 26 min 40% B. Compounds were identified by comparing retention times and the m/z values obtained by mass spectra and SRM modes, with the mass spectra and SRM modes from standards (ox bile) tested under the same conditions. The obtained results were compared with data from Scherer et al. 36.
Source: Ecology - nature.com


