AOB and NOB cultures were enriched from the pond sediments under autotrophic conditions with ammonia and nitrite as a sole energy source. For determining in vitro activity of the consortia, the established enrichments were spiked with known concertation of ammonia (19 ppm) and nitrite (16 ppm) as described in methods. The AOB enrichment efficiently oxidised ammonia to nitrite in 10 days while NOB consortium oxidized nitrite to nitrate in 14 days (Fig. 1). Though non-specific isolation media is available, it is a challenging effort to isolate slow-growing nitrifying bacterial species in pure form and are maintained as enrichments. Our enrichments being highly efficient in the oxidation of ammonia and nitrite, in the present study, 16S rRNA high throughput sequence methods were used for knowing the bacterial diversity in the AOB and NOB consortia, and the results revealed the presence of several unclassified lineages in these enrichments.
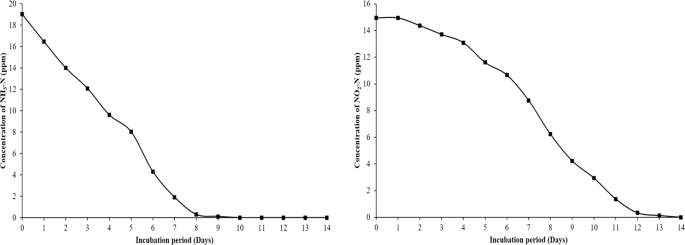
In-vitro Ammonia oxidation rate (a) and Nitrite oxidation rate (b) by AOB and NOB enrichments.
Diversity of microbial communities
In total, approximately 488,402 bacterial sequence tags from AOB enrichments and 384,579 bacterial sequence tags from NOB enrichments for 16S rDNA with an average length of 2 × 300 bp were obtained. Following sub-sampling, a total of 2,948 OTUs for AOB and 1,069 OTUs for NOB consortia respectively were obtained at 97% similarity index. Shannon alpha diversity index was found to be 7.64 in AOB enriched culture and 4.85 in NOB consortia confirming rich microbial diversity among the enrichments (Table 1). Comparative analysis showed that a total of 887 (21.92%) OTUs were common among AOB and NOB consortia (Figs. 2, S1).
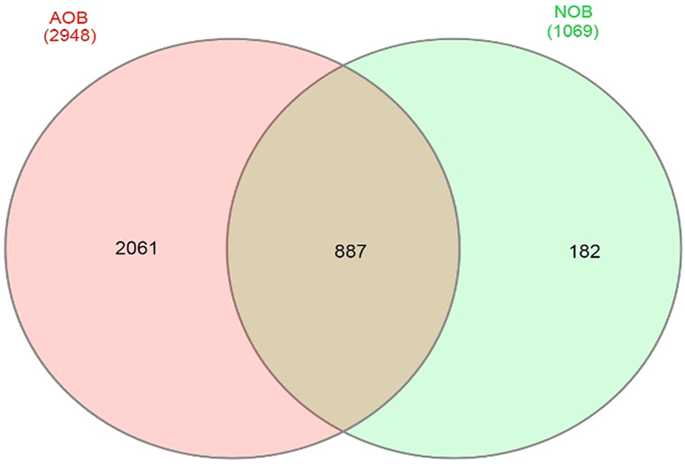
Venn diagram showing number of common OTUs between AOB and NOB consortia.
Relative abundance of bacterial community
With 16S rDNA high throughput sequencing, nearly 4,000 OTUs were recovered from the two-distinct nitrifying bacterial enrichments. Altogether a total of 47 bacterial phyla were observed in both the enrichments. Eubacteria dominated both AOB and NOB consortia (96% and 99% respectively) with remaining taxa belonging to Archaea. The proteobacterial phylum was found to be dominant in both the enrichments, with 31.46% and 39.75% in AOB and NOB consortia respectively. Additionally, Acidobacteria, Actinobacteria, Bacteroidetes, Chloroflexi, Cyanobacteria, Firmicutes, Nitrospira and Planctomyceteswere were the other phyla present in both AOB and NOB enrichments. Among the proteobacterial phylum, α-Proteobacteria dominated in AOB (20%) and NOB (18%) consortia while γ proteobacteria constituted 16% and 8% in NOB and AOB consortia respectively (Figs. 3a, 4).
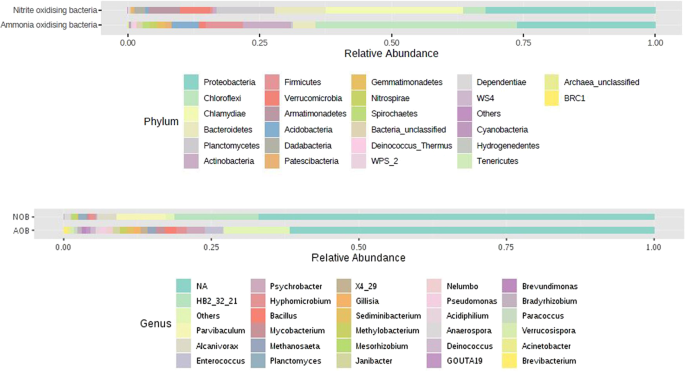
Phylum (a) and genus (b) level distribution among AOB and NOB enrichments.
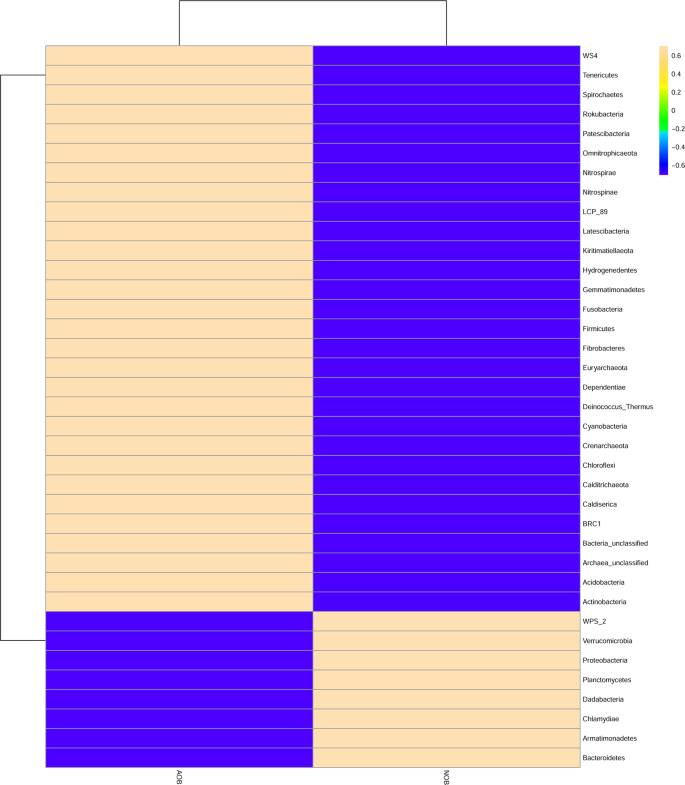
Dominant taxa present in AOB and NOB consortia at the phylum level.
Taxonomic composition of microbial enrichments
Microbial communities in ammonia oxidising enrichment
A total of 2,948 species were observed in AOB consortia, out of which two sequences were assigned to AOB group belonging to Nitrosomonas, and Nitrosococcus genera and two belong to archaeon group comprising Nitrosopumilus and Nitrososphaera genera. Of the OTUs belonging to AOB guild, 20 OTUs were identified as Nitrosomonas marina. The enrichments also contained 107 OTU s of Nitrosococcus genera (Fig. 5), represented under unclassified species clade. Among the ammonia oxidising archaea group, eight OTUs belong to Nitrosopumilus comprising unclassified species and four OTUs belonging to Nitrososphaeraea comprising the Candidatus Nitrososphaeraea gargensis (Fig. 5). The genus Stanieria belonging to the group of methyl ammonia oxidiser community were also found (four OTUs). Similarly, 269 OTUs showing similarity to Nitrospira-like AOB organisms were observed in the consortia. Genera with lesser abundance belonging to Psychrobacter, Bacillus (two OTUs), Hyphomicrobium (two OTUs), Methanosaeta (two OTUs), Sediminibacterium (one OTU), Pseudomonas (four OTUs) and Candidatus species (six OTUs) were recorded (Fig. 3b, Table S1).
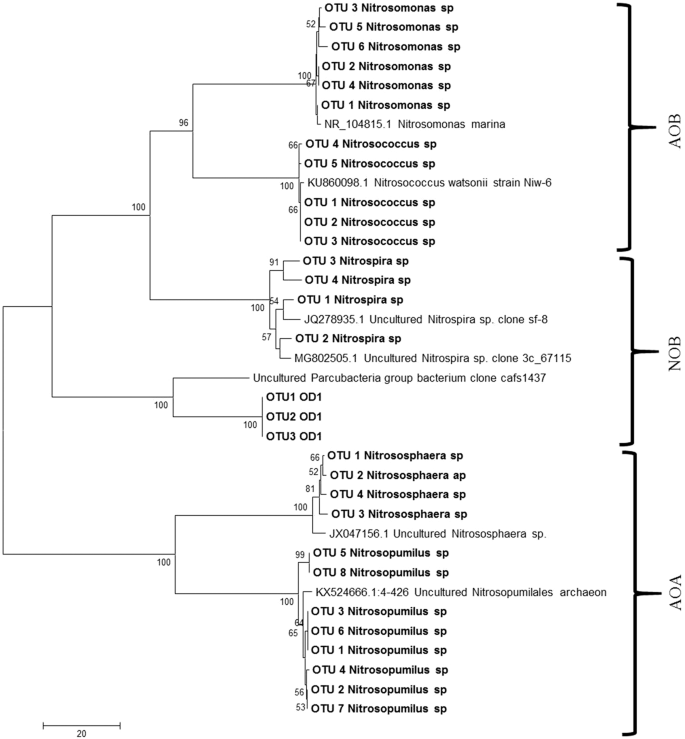
Phylogenetic analysis of representative sequences of Ammonia oxidising bacteria, archaea and nitrite oxidising bacteria in AOB and NOB enrichments.
In our study, two types of aerobic autotrophic microorganisms were observed in the AOB enrichments such as Nitrospira and Nitrosomonas that are known to be major players of ammonia oxidation. Autotrophic microbes can grow till exhaustion of nutrients and remain dormant for a certain period of time. Though heterotrophic microbes can grow five times faster than autotrophs, the latter group of microbes have significantly higher nitrification efficiency21. The AOB strains obtained in our study were assigned to the Nitrosomonas lineage (Fig. 5), confirming with the previous studies that these bacteria in this lineage can survive in low ammonia concentration, in soils9, lake sediments22 and biofilters23. Apart from ammonia oxidising bacterial population, archaea bacterial population were also detected in the developed enrichments. The majority of AOA classified till now were found to be oligotrophic and grow under low ammonia concentration. Gao et al.24 showed that AOB were more competitive than AOA under high concentration of ammonia. Similarly, substrate inhibition of archaeal nitrification under high concentration of ammonia has been reported25,26,27,28. Distribution of AOA is also ecosystem dependent, for example, Ca. Nitrosopumilus is widely present in seawater29 while Ca. Nitrososphaera more abundant in soil habitats with a wide range of pH and also in different aquatic ecosystems30. The results obtained in this study also showed lower diversity of AOA than AOB confirming with the previous studies. Phylogenetic analysis of archaea bacterial sequences were clustered into Nitrosopumilus and Nitrosophaera group and showed a distinct lineage towards ammonia oxidising bacterial groups (Fig. 5).
From our findings, the majority of microbes identified were from Proteobacteria phylum that is widely present in natural environments and plays a vital role in nutrient cycling and mineralisation of organic compounds31. Nearly 62% of the identified sequences were found to be unclassified genera (Fig. S2) indicating there might be novel microbial genera involved in ammonia oxidation or involved in aiding ammonia oxidation process. The dominant genera of ammonia-oxidising nitrifiers such as Nitrosomonas sp., Nitrosomonaseuropea and Nitrospira sp. have been documented in this study are responsible for efficient nitrification. Members of the group Nitrospira which is known to be a complete ammonia oxidizer32 is also found in our enrichments.
The Limnohabitans spp. present in AOB consortia is well-studied opportunistic bacterial group, with rapid generation time, acting on low molecular weight dissolved organic matter33. A recent study showed Limnohabitans species, strains Rim28 and Rim47 had great metabolic versatility, including photosynthesis, autotrophic carbon fixation, and ammonium and sulphur oxidisation34. Fitzgerald et al.35 reported the involvement of Luteibacter spp. of Xanthomonadaceae family in autotrophic utilisation of ammonia as a sole source of nitrogen in the low DO-nitrification process. Along with Nitrosomonas, Nitrosopumilus and Limnohabitans in AOB consortia these groups can also play a role in oxidising ammonia into nitrite.
Heterotrophic bacterial cultures like Bacillus, Arthrobacter, Pseudomonas and Exiguobacterium belonging to various phyla such as Firmicutes, Actinobacteria and Proteobacteria were observed in the enrichments and may not specifically be ammonia-oxidising nitrifiers; probably from the source water used for raising the enrichment. The results obtained in our study show that the enrichments consist of bacteria having mixotrophic ammonia oxidation and are well corroborated with the previous studies36 (Fig. S3).
Microbial communities in nitrite-oxidising enrichments
A total of 1,069 OTUs were observed in the NOB enrichments of which 184 OTUs belonging to phylum Nitrospirae were detected predominantly, two families belonging to Nitrospiraceae and Thermodesulfovibrionaceae. Under Nitrospiraceae 56 OTUs belong to unclassified Nitrospira genera whereas, 36 OTUs of Thermodesulfovibrionaceae families were distributed into three unclassified genera. NOB enrichments had 60% of unclassified sequences (Fig. S2). One of the interesting findings in NOB consortia is the presence of superphylum OD1. A total of 17,961 sequences belonging to the phylum OD1 has been classified as superphylum Candidate Phyla Radiation (CPR) Parcubacteria (Fig. 5) group which was identified as a major player in marine nitrogen cycle37.
In this study, we have documented a diverse lineage of Nitrospirae phylum known to play a major role in the nitrite oxidising process. To the best of our knowledge, a few chemolithoautotrophic microbes with nitrite oxidation have been documented. Most of these groups were previously documented to play a major role in nitrite oxidation and also involved in the denitrification process with key genes involved (Table S1). For the nitrite oxidisers, eight OTUs related to Nitrospira were detected in the enrichment. Nitrospira (Fig. 4) is identified as the most abundant nitrite oxidisers in low-nitrite environments38,39. A poorly characterised taxon, 0319-6A21 (Table S1) belongs to Nitrospira was first reported from lava caves than in surface soils40. Organic compounds produced by both AOB and NOB serve as a substrate for heterotrophic microbial growth41 whereas, a Nitrospira population can feed on the dead heterotrophic community during the enrichment in mineral nitrite medium42. NOB may also benefit from the presence of heterotrophs, for example, via heterotrophic nitrate reduction.
The previous study has demonstrated that members of Proteobacteria can utilise reduced sulphur compounds to obtain energy and also through carbon assimilation via the reductive tricarboxylic acid (rTCA) cycle43. Interestingly rare taxa <1% belong to Armatimonadetes, AC1, NC10, NKB19, OP8 and GN04 (Table S1) were detected in both the enrichments. Presence of NC10 group in both the samples suggests their involvement in methane oxidation coupled with nitrification process44,45 (Fig. S3). Although these groups were found to be present in less number, previous reports have also predicted their involvement in nitrogen cycle coupled to some other biogeochemical pathways.
Heterotrophic nitrate-reducing bacterial communities
The traditional theory of biological nitrogen removal makes a rigid difference between nitrification and denitrification process based on distinct growth conditions of nitrifiers and denitrifiers; however, heterotrophic nitrification and aerobic denitrification processes can simultaneously occur46,47. The potential for nitrification and denitrification was detected in both autotroph and heterotroph microbial lineages, suggesting the involvement of a diverse range of nitrogen metabolic pathway. Heterotrophic nitrifying bacteria produce hydroxylamine, nitrite and nitrate by nitrification process using organic carbon as a source for their growth. Most of these bacteria are capable of converting nitrification products directly to nitrogen gas through the process of aerobic denitrification48. In this study other than autotrophic microbes, heterotrophic microbes were also predominantly present in both the enrichments. Proteobacteria were the most critical contributors of all genes involved in denitrification pathway, besides the presence of other groups like Nitrospirae, Bacteroidetes, and uncultured microorganisms were shown to play essential roles in denitrification. The dominant denitrifiers in our enrichments mainly include Alcaligenes, Pseudoxanthomonas, Pseudomonas, Marinobacter, Shewanella, Thalassospira and Rhodobacter (Table. S1), most of which are taxonomically affiliated with ß or γ proteobacteria and bacteroidetes49. Proteobacterial groups, found in both the enrichments, were closely related to Hyphomicrobium, Paracoccus, Pseudomonas and Comamonas spp. (Table S1, Fig. S3) and known to be denitrifiers50. Studies by Feng et al.51 showed that bacteria belong to Bacillus, P. putida, P. stutzeri, Hydrogenophaga, and Achromobacter have been shown to have nitrification and aerobic denitrification abilities
The heterotrophic genera identified in AOB enrichment belong to Burkholderia, Exiguobacterium, Methanoregula and Methanosaeta while, groups belonging to Aequorivita, Candidatus Entotheonella, Erythrobacter, Flavobacterium, Idiomarina, Mesorhizobium, Oleibacter, Parvibaculum were present in NOB enrichments. Groups belonging to Alcanivorax, Anaerospora, Arenibacter, Bacillus, Bradyrhizobium, Brevibacterium, Brevundimonas, Candidatus Solibacter, Desulfovibrio, Devosia, Halorhodospira, Hyphomicrobium, Lewinella, Planctomyces, and Sphingobium were present in both AOB and NOB enrichments (Table S1, Fig. S3). These groups present in enrichments were classified based on their predicted functional properties such as denitrification, nitrification and nitrate reduction using KEGG orthology database (Fig. 6, Table S1). In this study, a broader diversity of metabolic genes involved in nitrogen metabolism, viz. nitrification, denitrification, nitrogen fixation, and dissimilatory nitrate reduction to ammonia (DNRA) were predicted using PICRUSt analysis in both the AOB and NOB enrichments.
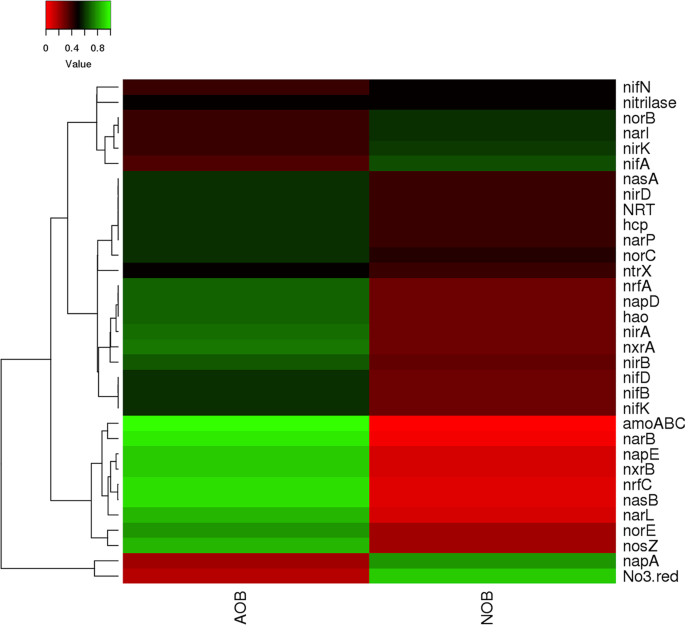
Heat map showing the distribution of genes involved in nitrogen cycle based on KEGG orthologous gene identification by PICRUSt analysis.
Many OTU’s belong to yet to be classified group described as Candidatus species were also found to be predominant in both AOB and NOB consortia. Based on recent findings, AOB and NOB enrichments contain bacterial isolates belonging to super-phylum Verrucomicrobia, which are involved in methane regulated pathway52 were documented in both the enrichments. Some of the notable groups, such as Natronococcus, known for nitrate reduction were present in both the enrichments. Other Candidatus groups belong to unclassified Lautropia, phylum BRC2, Koribacter, Solibacter, Aquiluna, and Entotheonella (Table S1)12,53 were also found to be denitrifier strains. Few groups belong to Methanoregula, and Candidatus Hydrogenedens54 were found to convert nitrate to ammonia through dissimilatory nitrate reduction pathway.
16S rDNA high throughput gene sequencing indicated that the nitrifying populations in the enrichment involve a Nitrosomonas-like Nitrospira-like AOB, Nitrosopumilus, Candidatus Nitrososphaera and Nitrospira-like NOB that are adapted to ammonia and nitrite condition, respectively. Previous studies have also reported the heterotrophic nitrification and ammonification pathway present in these groups, but their mode of function remains less known. Based on preferential enrichment, and the reports from existing literature, organisms belong to Pseudomonas, members of the family Xanthomonadaceae, Limnohabitans, and Sphingomonas, CPR OD1, Candidatus species of Opitutus, Staneria, and Exiguobacterium group have the potential to participate in ammonia as well as nitrite oxidation, can function either as heterotrophic nitrifiers, or via autotrophic nitrification through yet uncharacterized pathways33,35,37. The potential for nitrification and denitrification was detected in a diverse lineage of both autotroph and heterotroph microbial communities, suggesting a diverse range of potential involved in nitrogen metabolism. One such group observed in the enrichment is Sulfurimonas, which reduces nitrate to dinitrogen gas coupling sulphur oxidation to denitrification pathway has been previously documented by Cerqueira et al.55.
Presumptive functional profile of microbes in the enrichments using PICRUSt analysis
PICRUSt was used to predict the function of enrichments based on 16S rDNA and the functionalities obtained are predictive. The analysis predicted 24 different nitrogen cycling genes that are involved in nitrification, denitrification, ammonia and nitrogen transporter family, nitrate reduction and ammonia assimilation (Fig. 6, Table S1). Genes coding for the enzyme involved in nitronate monooxygenase, nitrile hydratase, nitrate reductase, nitrilase, nitric oxide dioxygenase, nitric oxide reductase, nitric-oxide synthase, nitrite reductase, nitric-oxide reductase, nitrogenase, nitric nitrogen fixation protein, nitroreductase/dihydropteridine reductase, nitrous-oxide reductase, nitroreductase, nitrate reductase, nitrogenase, nitric oxide reductase, which are distributed among several phyla were predicted. Most dominant gene cluster involved in nitrification, denitrification, DNRA and assimilatory nitrogen reduction is given in Table (S1). Other than nitrogen metabolism, metabolic pathways related to sulphur, carbon, propionate, TCA cycle, amino acid and sugar metabolism, biodegradation and carbon fixation pathways and pathways associated with the degradation of aromatic compounds, nitrotoluene degradation and polyaromatic hydrocarbon degrading groups were also predicted to be present in both the enrichments (Figs. 7 and S4). However, it is to be noted that use of function prediction tools relies mainly on the availability of reference genomes included in the algorithm56 and the predicted functions are indicative, which needs further confirmation.
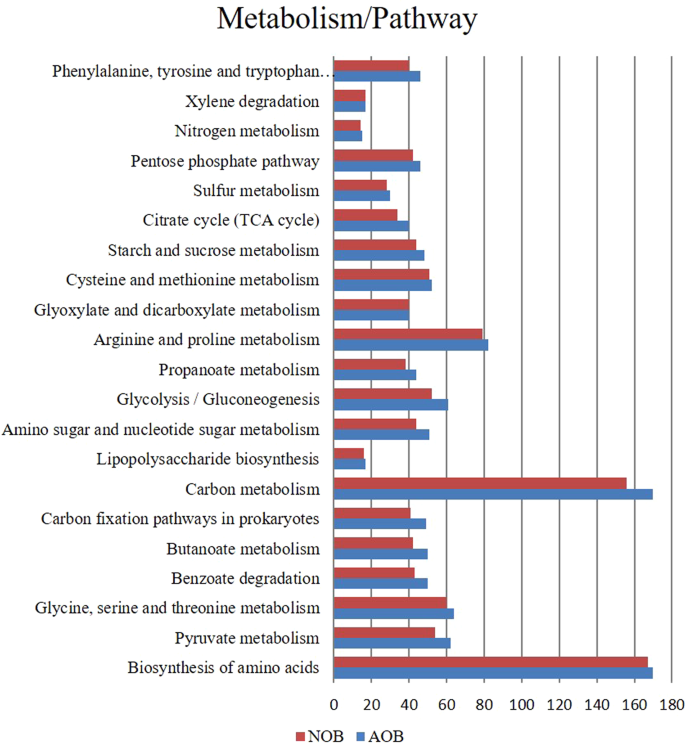
The general metabolic pathways of AOB and NOB consortiaby network enrichment analysis.
Due to the presence of many unclassified genera in the enrichment, this study suggests that heterotrophic bacterial group documented in the enrichment may play a role in nitrogen conversion. From the findings, we believe presence of heterotrophic bacteria along with autotrophic bacterial groups and form a microcosm57. This may be due to production of the secondary metabolites, including nitrogenous compounds by autotrophs facilitating survival of the heterotrophic bacteria in the enrichments. Recently nitrification activity has been observed in heterotrophic bacteria, which are capable of utilising ammonia and nitrite33,36,47. Although nitrifying bacteria may act as primary producers and play a significant role in the nitrogen cycle, little is known about its adaptability with heterotrophic microbial community and mode of interactions.
Source: Ecology - nature.com


