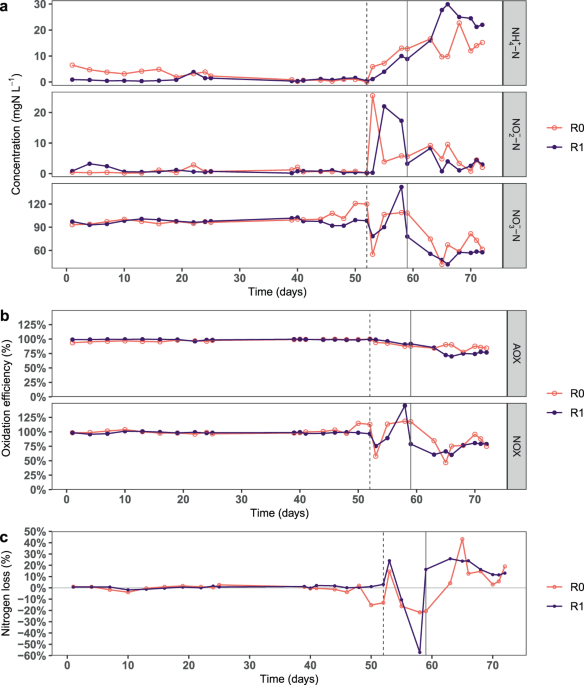
In phase 1, both reactors had similar (~100%) nitrification performance. While this is consistent with a previous study7, it contradicts other studies that reported a 30–70% reduction in AOX at C/N ratios of 0.6-0.9 16,34,35. The discrepancy could be because in some of these studies, the same bioreactor was operated at new C/N ratios with 2-4 weeks of acclimation, whereas in the present study, two separate bioreactors were adapted to different C/N ratios for four months. Thus, over an extended period, carbon-fed nitrifying bioreactors can adapt and perform as well as autotrophic bioreactors. Despite similar activity during phase 1, the microbial community composition in the two treatments was significantly different, indicating that the C/N ratio had a deterministic effect on the microbial succession in the biofilm – including the nitrifying bacteria. The succession may have also been influenced by the biofilm thickness, as higher carbon supply leads to thicker biofilms 8,36,37,38. As the thickness influences the biofilm properties in several ways, such as oxygen diffusion and redox gradient, it can play an important role in determining the microbial community composition14,39. Thus, both the biofilm thickness and microbial composition likely influenced the response of the bioreactors to increases in carbon supply.
An acute increase in the dissolved organic carbon inhibited nitrification 10 times more severely in the carbon-fed reactor R1 than in R0. The nitrifying biomass in both reactors was likely equal, as the nitrification activity during phase 1 was similar. However, R1 had higher heterotrophic biomass than R0 due to the greater proportion of heterotrophs. Thus, the increase in acetate concentration stimulated heterotrophic growth to a greater extent in R1. This intensified the competition for oxygen and space, thereby inhibiting the nitrification activity more severely in R1 than in R0. To the best of our knowledge, this is the first study to model the impact of acute carbon stress on nitrifying biofilms operated at different C/N ratios. The results suggest that autotrophic conditions should be maintained in the nitrification units of water treatment systems receiving influents with varying carbon concentration, to prevent inhibition of nitrification by carbon fluctuations. Autotrophic conditions may be maintained by designing pre-treatment systems for carbon removal before the nitrification unit. Other important design variables are the loading rate per biofilm area and the oxygen supply. Apart from inducing competition, organic carbon can also directly inhibit nitrification by inactivating enzymes in the nitrification process15,35. But in such a scenario, we would have expected similar inhibition in both reactors, unlike in this study. The greater inhibition in R1 strongly suggests that it was due to resource competition between the nitrifiers and the heterotrophs rather than direct inhibition by organic carbon.
Under a chronic increase in carbon supply in phase 3, the AOX decreased by 14–22% (Fig. 1b). R0 had higher nitrification efficiency than R1 despite a lower proportion of nitrifiers, likely due to greater functional redundancy. The decrease in nitrification efficiency with increasing carbon supply is consistent with previous studies7,9, but in contrast to the 70% reduction reported by another study34. Further, nitrite accumulation was observed in phases 2 and 3 (and the acute inhibition tests), suggesting that despite different dominant NOB in the two reactors, the NOB were more suppressed than the AOB. This was likely because the bacterial growth was oxygen-limited. As NOB have lower specific growth rates and oxygen affinity than AOB, they would be inferior in the competition for oxygen with AOB and the prolific heterotrophs3,40. This observation is in contrast to a previous study where nitrite accumulation was not observed35, but consistent with some other studies16,41. In the beginning of phase 3, full carbon removal was achieved in R0 within 24 hours, indicating that autotrophic biofilms adapt quickly to consume organic carbon. This is important when designing systems where influents may have variable carbon concentration.
A sustained increase in dissolved organic carbon supply also increased denitrification activity in both reactors. On the first days of phases 2 and 3, denitrification activity was significantly greater in R1. Also, in phase 3, the long-term denitrification activity (nitrogen loss) was slightly greater in R1 (18 ± 6%) than in R0 (10 ± 6%) (excluding day 65). The values are close to a previous study that reported 18–31% nitrogen loss at low C/N ratios (0.6–2.3)16. The higher denitrification activity in R1 may be linked to the greater thickness of carbon-rich biofilms and more oxygen competition in R1. Under oxygen limitation, anoxic processes such as denitrification may be favoured in the presence of organic carbon14,42. The denitrification strategy can alleviate the competitive pressure by using nitrate instead of oxygen for respiration and by reducing the carbon available for the aerobic heterotrophs (thereby slowing growth and reducing the oxygen consumption). Therefore, under the carbon- and nitrate-rich conditions in phase 3, heterotrophs that could utilize both oxygen or nitrate for respiration had a competitive advantage, especially in the oxygen-deprived layers of the biofilm. This is also reflected in the change in the species inventory of the biofilm, as the relative abundance of potential denitrifiers – Acinetobacter (in R0 only), Delftia, and Acidovorax16,43,44– increased substantially (total proportion up from <1% to 30–50%). As mentioned previously, simultaneous nitrification and denitrification can be problematic in some systems. Under low nitrate and high sulphate concentration (especially in seawater), sulphate reducing bacteria (SRB) can produce H2S, a health hazard in WWTP17 and a huge problem in marine RAS, where even low concentrations of H2S can kill the fish. In the past couple of years, H2S has been suspected as the cause of mass mortalities of fish in brackish and seawater RAS. In the present study, SRB were not detected at the assigned sequence similarity cut-off. Nonetheless, it is advisable to maintain low carbon concentration in RAS bioreactors to avoid anoxic zones in the biofilm and minimize the risk of H2S.
In contrast to a previous study16, the biofilm α-diversity (both overall and nitrifying OTUs) in the carbon-deprived reactor R0 was higher than in R1 and reduced upon increasing carbon supply. This suggests the loss of K-strategists that could not compete with r-strategists at a higher carrying capacity. By comparison, the diversity in R1 did not change during the study, even though the nitrification efficiency decreased and denitrification activity increased. The proportion of nitrifiers decreased with increasing C/N, corroborating previous studies7,15,37. In phase 1, the proportion of AOB in carbon-fed R1 was only 5% compared to 15% in R0, likely due to the competition for space and oxygen. Although the presence of nitrifiers throughout the biofilm have been reported12,40,45, the AOB Nitrosomonas generally prefer the oxygen-rich outer biofilm layers14,42. However, in the carbon-fed biofilm, heterotrophs likely dominated the outer biofilm, with the AOB underneath and the NOB in the layers below20,36,38,46. As the deeper strata are usually oxygen limited40,42, microbes with a higher oxygen affinity should have been dominant in this niche. So the prevalence of N. oligotropha-like taxa in R1 is surprising, as this lineage is reported to have a low oxygen affinity compared to other Nitrosomonas species, such as N. europaea/eutropha47,48. However, the dominant AOB in R1, N. urea and N. oligotropha, both have a higher ammonia affinity than N. europaea/eutropha, which may have made them superior competitors based on substrate affinity in the diffusion-limited biofilm layers49,50. By comparison, in the autotrophic biofilm, the dominant OTUs were similar to N. oligotropha/aestuarii/marina and N. europaea. These two taxa likely dominated different biofilm niches, as shown by another study where N. europaea-lineage and N. oligotropha coexisted on the outer biofilm layer, whereas N. oligotropha dominated the deeper layers51. Moreover, N. europaea was reported to support heterotrophic growth and biofilm formation in the absence of external organic carbon52. Thus, the greater oxygen availability in R0 selected for different taxa as opposed to oxygen-limited R1. Our results are in contrast to another study16, where the dominant AOB (N. europaea) did not change with C/N ratio, perhaps because of lesser adaptation time to the C/N ratio.
In phase 1, the dominant nitrite oxidizer in R0 belonged to the genus Nitrobacter. The r-strategist Nitrobacter likely outcompeted Nitrospira owing to the lesser oxygen competition (hence higher oxygen availability) in the carbon-deficient reactor42,53,54. Nitrobacter were also identified as the dominant NOB at low C/N ratios (0–2.3) in a previous study16. However, other studies did not detect Nitrobacter in autotrophic biofilms13,20,40, suggesting that the initial microbial community composition may influence the steady-state community55,56. By contrast, Nitrospira was the dominant nitrifier in R1, present at a relative abundance as high as 20%. Nitrospira preferentially reside in the interior of the biofilm, and being K-strategists, are more competitive than Nitrobacter under oxygen-limiting conditions40,42,54,57. The microaerophilic nature of Nitrospira may, therefore, have made it a superior competitor to Nitrosomonas and Nitrobacter in R1. Moreover, the higher proportion of NOB than AOB in R1 suggests that the Nitrospira may have grown by comammox or alternative growth mechanisms, which can be advantageous in ammonia-limited environments58,59. The ability of Nitrospira to co-exist with heterotrophs may also explain why it is wide-spread in natural ecosystems and is the dominant nitrite oxidizer in wastewater treatment plants58. Indeed, upon increasing the carbon supply, the proportion of NOB reduced greatly in R0, with a significant decrease in the proportion of Nitrobacter but a slight increase in the proportion of Nitrospira. In contrast, the increased carbon supply did not affect the NOB composition in R1.
Phase 2 was run for only seven days, which may be insufficient to confirm the long-term impact of the increased C/N. The fluctuations in ammonia, nitrite, and nitrate in the end of phase 3 suggest that steady state was not achieved, especially in R0. This indicates that the destabilizing effect of a chronic increase in carbon supply persists for at least two weeks. Also, the results in phase 3 were the combined effect of the increased carbon and nitrogen loading rate8,12, and decreased HRT6. In this study, the differences between the treatments were likely due to differences in C/N as well as the biofilm thicknesses. To unconfound biofilm thickness and C/N ratio, future studies should compare the impact of organic carbon on biofilms with controlled thickness, such as in Z-carriers11.
In conclusion, this study showed that autotrophic bioreactors were 10 times less inhibited by acute increase in organic carbon than carbon-fed bioreactors – likely due to differences in the heterotrophic potential. A sustained increase in carbon supply and substrate loading reduced nitrification efficiency and increased denitrification to a greater extent in carbon-fed biofilms. The chronic increase in carbon supply also increased the proportion of potential denitrifiers in both reactors. The results suggest that autotrophic conditions are preferable in nitrifying bioreactors when acute carbon fluctuations are expected and/or anoxic processes are undesirable, such as in RAS. This implies that nitrification systems should be designed to maintain high nitrifying to heterotrophic biomass ratio, for e.g., by pre-treatment to remove organic carbon before the nitrifying unit.
Source: Ecology - nature.com


