Data collection
Bats were encountered in day roosts within SchweglerTM woodcrete boxes distributed across our study site, Wytham Woods (51°77′N, 1°33′W) or caught at night in mist nets and harp traps in the same locality. All catching, handling and ringing of bats was carried out under project licence from Natural England (2018-36143-SCI-SCI and preceding licences). All bats were returned to their roost, or released at the site of capture, as soon as possible following examination.
Assessment of reproductive condition
The presence or absence of enlarged testes and distended caudae epididymides (swelling being indicative of spermatogenesis and sperm storage respectively), was recorded based on visual assessment by experienced surveyors during manual handling of bats.
Males were classified into four categories of reproductive condition38: (A) non-reproductive (testes not swollen, epididymides not distended), (B) testes swollen, epididymides not distended, (C) testes swollen, epididymides distended, and D) testes not swollen, epididymides distended, see Supplementary Fig. S2). In male bats there is a progression from A > B > C > D (a process taking 1–2 months per individual once commenced, based on 271 within-season recaptures of 105 males that were observed to progress through stages of spermatogenesis) per breeding season in all reproductively active males.
Assessment of whether or not individual males were in reproductive condition each season was based on any encounter where swollen testes and/or epididymides (reproductive condition B, C or D) were observed during June to October. Encounters with adult males with distended epididymides but without enlarged testes in spring (n = 27 bats on 30 occasions during April and May) were excluded in an attempt to record the temporal occurrence of spermatogenesis per annum (i.e. enlargement of the testes due to spermatogenesis prior to distension of the caudae epididymides due to sperm storage), and not vestiges of the previous breeding season. Only males encountered during September or October in non-reproductive condition (A) were classified as non-breeders that season. Individuals encountered only during April to August in non-reproductive condition (A) were classed as ‘unknown’ breeding status for that season, as they might still have come into reproductive condition later whilst not observed. Some individuals found in non-reproductive condition (A) during August were recaptured in reproductive condition (B or C) during September.
Age assessment
Males were classified as juvenile (actual age = 0) based on unfused finger joints39 or a suite of secondary characteristics indicative of bats born that season, including ‘fresh’ wing membranes, grey pelage, dense ‘chinspot’, dark tunica vaginalis, and light weight18,32,40,41, once the epiphyseal gaps were fully ossified. Adult males (minimum age = 1) were identified on first capture based on timing (encountered early in the season before juveniles were full-grown), evidence of prior reproduction such as elongation of the caudae epididymides or unpigmented tunica vaginalis, and colouration or weight15. Bats of indeterminate age, possessing an ambiguous mixture of juvenile and adult characteristics, were classified as ‘unknown’ (minimum age = 0) when ringed. The majority of data for adult males (2324/2693 = 86.30% of M. daubentonii encounters, and 807/887 = 90.98% of M. nattereri encounters) were derived from confirmed adults (i.e. ringed individuals recaptured 1–12 years post-ringing).
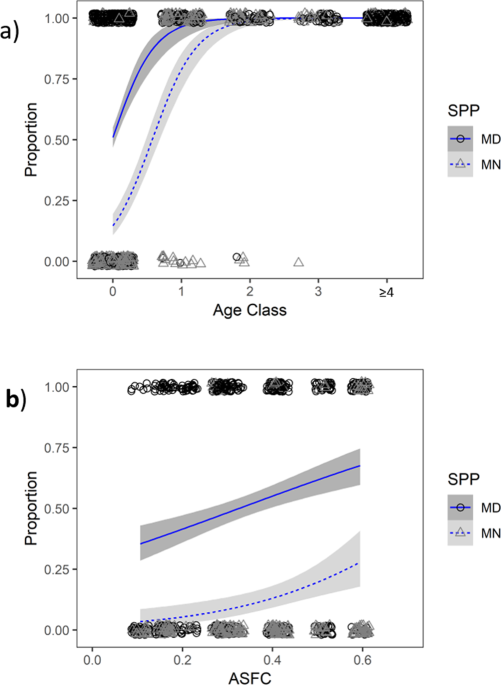
The proportion of males in reproductive condition per age class was based only on individuals of known (actual) age for age classes 0, 1, 2 and 3 (ringed as juveniles and recaptured 0–3 years later), age class ≥4 included bats of known and minimum age (ringed at least four years before as a juvenile or ‘unknown’, or at least three years earlier as an adult).
Biometric measurements
Forearm length was measured to the nearest 0.1 mm using dial calipers (n = 3779, 1–24 measurements per male). Body mass was recorded to the nearest 0.1 g using a spring balance (n = 3598, 1–22 measurements per male). Body mass measurements obtained from males captured at night were excluded from our analyses as during foraging bouts substantial weight gain can occur due to ingested prey.
Data Analysis
Model I: Reproductive condition in juvenile males
The proportion of juvenile males reaching puberty was treated as a binary response variable (Y = found in reproductive condition (B, C or D) as a juvenile, N = found in non-reproductive condition (A) during September or October in the year of their birth) in logistic models, using the ‘glmer’ function in package lme442 in R version 3.5.343, with species (SPP = Myotis daubentonii, abbreviated to Md, or M. nattereri, abbreviated to Mn) as a categorical explanatory variable, and spring weather conditions during their birth year (ASFC which is a proxy for breeding phenology and birth timing14) as a continuous explanatory variable. We also tested for an interaction between SPP:ASFC in case the effect of spring phenology varied between species (details of model selection are presented in Supplementary Table S2). Year as a factor (fYR) was included as a random intercept effect to provide the correct level of replication.
Model II: Does body mass vary with age and reproductive condition?
Due to the temporal spread of body mass (g) and reproductive condition (A, B, C and D) data throughout the summer survey season (see Figs. 2a,b and 3) it was not possible to incorporate weight as an explanatory variable in an analysis of reproductive condition as determining cause and effect between body mass and reproductive condition was problematic (i.e. the weight of males in non-reproductive condition A may influence the likelihood and timing of spermatogenesis, but the body mass of males in reproductive condition B and C may have been influenced by energy expenditure during spermatogenesis). We therefore used body mass as the response variable in linear mixed models, using the ‘lmer’ function in R package lme442, with SPP, and age class and breeding status (ACL_BS with four categories; J_NB = juvenile non-breeder, J_RC = juvenile achieving puberty, A_NB = adult non-breeder that season, and A_RC = adult in reproductive condition that season) as categorical explanatory variables, and day of the year (DOTY), ASFC and forearm size (FA) as continuous explanatory variables. We also tested for interactions between predictor variables in case the effect of ACL_BS varied between SPP, or in case the effects of DOTY and ASFC varied between SPP, ACL_BS, or in relation to FA, an interaction between DOTY and ASFC was also tested (see Supplementary Table S3 for details of model selection). Because these datasets contained repeat measures (individuals were observed on between 1–8 occasions per annum, for 1–13 breeding seasons) both individual (IND = ring no. a unique identifier per individual) and fYR were included as random intercept effects to give the correct level of replication. Based on recent recommendations33 we did not correct body mass for forearm size. Due to visual examination of Fig. 2c,d, however, FA was included as a predictor variable.
Model III: Phenology of reproductive condition in relation to species and age
Cumulative Link Mixed Models (CLMMs) with the progression of reproductive condition (A > B > C > D) on an ordinal scale as our response variable were performed using the ‘clmm’ function in package ordinal44 in R. SPP and age class (ACL with three categories; juveniles, young adults (aged 1–3 years), and older males (aged ≥4 years old), were included as categorical explanatory variables. DOTY and ASFC were included as continuous explanatory variables. We tested for differences in the temporal distribution of reproductive phases by including interaction terms between DOTY, SPP and ACL. We also tested for interactions between ASFC and the other predictor variables (see Supplementary Table S4 for details of model selection). IND and fYR were included as random intercept effects to provide the correct level of replication. Only bats known to have commenced spermatogenesis that breeding season were included in the CLMM dataset.
Model selection
Akaike Information Criterion corrected for small sample size (AICc) and model weights were derived using package AICcmodavg45 in R, and were used to rank model sets (details of model selection are provided in Supplementary Tables S2–S4) in accordance with an Information-Theoretic approach46. Results from the favoured model in each set are presented in Table 1.
Ethical approval
All catching, handling and ringing of bats was carried out under project licence from Natural England (2018-36143-SCI-SCI and preceding licences). These methods were carried out in accordance with relevant international, national and institutional guidelines and regulations.
Source: Ecology - nature.com


