Ethics approval and consent to participate
This study used human blood samples to prepare mosquito artificial infectious blood meals. Methods were carried out in accordance with relevant guidelines and regulations, and experimental protocols were approved by the French Ethical Committee Ile-de-France I. Healthy donor recruitment was organized by the local investigator assessment using medical history, laboratory results and clinical examinations. Biological samples were supplied through participation of healthy volunteers at the ICAReB biobanking platform (BB-0033-00062/ICAReB platform/Institut Pasteur, Paris/BBMRI AO203/[BIORESOURCE]) of the Institut Pasteur to the CoSImmGen and Diagmicoll protocols, which have been approved by the French Ethical Committee Ile-de-France I. The Diagmicoll protocol was declared to the French Research Ministry under reference DC 2008-68 COL 1. All adult subjects provided written informed consent.
Mosquitoes
Experiments were carried out with a laboratory colony derived from a sylvatic population of Ae. malayensis (7th generation) collected in March 2017 and subsequently maintained at the Institut Pasteur du Laos. The Ae. malayensis colony was initiated with mosquito larvae collected along the Nam Noy River (17.768548°N, 105.381989°E) in the Nakai Nam Theun National Protected Area (NNT NPA), known as the Watershed Management and Protection Authority (WMPA), located in the Nakai district, Khammuane province, Laos13. A laboratory colony of Ae. aegypti (7th generation) maintained at the Institut Pasteur du Laos was used as a control. The Ae. aegypti colony originated in the town of Paksan (18.37134°N, 103.66586°E), Paksan district, Bolikhamxay province, Laos. Mosquitoes were reared under controlled insectary conditions (28 °C, 70% relative humidity, 12:12 hour light cycle) as previously described12. Eggs were hatched synchronously in a vacuum chamber for 1 hour. Larvae were reared in 24 ×34 ×9 cm plastic trays containing 1.5 L of dechlorinated tap water and fed with Tetramin (Tetra) fish food at a density of 400 larvae per tray. Eight hundred adults were kept in 30 ×30 ×30 cm Bugdorm-1 insect cages with permanent access to 10% sucrose solution. For olfactometer studies at the London School of Hygiene and Tropical Medicine, eggs from the Ae. malayensis and Ae. aegypti colonies were shipped to London and reared under similar insectary conditions.
Viruses
Vector competence experiments were carried out following previously described methods12 with low-passage DENV type 1 (DENV-1) and YFV isolates. The DENV-1 isolate (H15–3000) was originally obtained in 2015 through the Institut Pasteur du Laos DENV surveillance network in Vientiane capital, and was passaged three times in Aedes albopictus C6/36 cells before it was used in this study. Virus stocks were produced during the third passage following as previously described35. The YFV isolate (YFV-S79 strain) belongs to the West African lineage and was originally obtained in 1979 from the serum of a patient returning to France from Senegal36. Prior passage history of the YFV isolate included two passages in newborn mouse brains and two passages in C6/36 cells, which occurred before the isolate was obtained for this study. Virus stocks were produced during an additional C6/36 passage, as previously described for DENV35. Virus titration was performed by standard focus-forming assay (FFA) as previously described for DENV35 and YFV12. A commercial mouse anti-dengue virus complex monoclonal antibody (MAB8705; Merck Millipore) diluted 1:200 in phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin (BSA; Interchim) was used as primary antibody for DENV. A commercial mouse anti-flavivirus group antigen monoclonal antibody (MAB10216; Merck Millipore) diluted 1:1,000 in PBS supplemented with 1% BSA (Interchim) was used as primary antibody for YFV.
Oral challenge
Mosquitoes were orally challenged with DENV-1 in two separate experiments and with YFV in a third one, following previously described methods12,31. Briefly, 8-day-old females starved for 24 hours were offered an artificial infectious blood meal in 3 rounds of 15 minutes using a Hemotek membrane-feeding apparatus with porcine intestine as membrane. Blood meals consisted of a 2:1 mix of washed erythrocytes (rabbit erythrocytes for DENV experiments and human erythrocytes for the YFV experiment) and virus suspension. Human erythrocytes were obtained in accordance with relevant guidelines and regulations through a protocol approved by the French Ethical Committee Ile-de-France I, as described above. Adenosine triphosphate (Merck) was added as a phagostimulant37 to the blood meal at a final concentration of 10 mM. In DENV experiments, mosquitoes were exposed to 1.16–1.38 ×107 focus-forming units (FFUs)/mL of DENV-1. In the YFV experiment, they were exposed to 1.84 ×106 FFUs/mL of YFV. Fully engorged females were sorted on wet ice, transferred into 1-pint cardboard containers and maintained in a climatic chamber (28 °C, 70% relative humidity, 12:12 hour light cycle) with permanent access to 10% sucrose solution. Fourteen days after the blood meal, mosquitoes were cold-anesthetized to remove wings and legs for DENV experiments or paralyzed with triethylamine for the YFV experiment12 to collect saliva samples in vitro38. The proboscis of each female was inserted into a 20-µL pipet tip containing 5 µL of fetal bovine serum (FBS). After 30 minutes of salivation, head and body were separated and stored individually at −80 °C. The saliva-containing FBS was mixed with 45 µL of Leibovitz’s L-15 medium and immediately inoculated onto sub-confluent C6/36 cells for titration by FFA, as described above, without subsequent dilution.
Virus Detection
In DENV-1 experiments, bodies were homogenized individually in 300 µL of PBS. Body homogenates were centrifuged and viral RNA was extracted using the NucleoSpin RNA Virus kit (Macherey-Nagel) according to the manufacturer’s instructions. Detection of DENV-1 RNA was performed with the EXPRESS one-step Superscript qRT-PCR kit (ThermoFisher Scientific) using the following program: 30 min at 45 °C, 2 min at 95 °C, 55 cycles of 15 sec at 95 °C and 30 sec at 60 °C with a final step of 2 min at 25 °C. The 25-µL reaction volume contained 1x of reaction mix, 1.4 µM of primers, 5 µM of probe (forward: 5′-AAGGACTAGAGGTTAKAGGAGACCC-3′; reverse: 5′- CGWTCTGTGCCTGGAWTGATG-3′; probe: 5′-TCTGGTCTTTCCCAGCGTCAATATGCTGTT-3′)39 and 5 µL of RNA extract. In the YFV experiment, viral RNA was extracted by grinding bodies individually in 300 µL of squash buffer (Tris 10 mM, NaCl 50 mM, EDTA 1.27 mM with final pH adjusted to 8.2) supplemented with proteinase K (1 µL for 55.5 µL of squash buffer) and by incubating 100 µL of body homogenates for 5 min at 56 °C followed by 10 min at 98 °C. Detection of YFV RNA was performed using a two-step RT-PCR reaction to generate a 192-bp amplicon located in a conserved region of the NS3 gene of YFV. Total RNA was reverse transcribed into cDNA with random hexamers using M-MLV reverse transcriptase (ThermoFisher Scientific) using the following program: 10 min at 25 °C, 50 min at 37 °C and 15 min at 70 °C. The cDNA was subsequently amplified using DreamTaq DNA polymerase (ThermoFisher Scientific). The 20-µL reaction volume contained 1x of reaction mix and 10 µM of primers (forward: 5′-GCGTAAGGCTGGAAAGAGTG-3′; reverse: 5′-CTTCCTCCCTTCATCCACAA-3′)40. The thermocycling program was 2 min at 95 °C, 35 cycles of 30 sec at 95 °C, 30 sec at 60 °C, and 30 sec at 72 °C with a final extension step of 7 min at 72 °C. Amplicons were visualized by electrophoresis on a 2% agarose gel. In all experiments, mosquito heads were homogenized in 300 µL of Leibovitz’s L-15 medium supplemented with 2% FBS, centrifuged, and assayed by a qualitative version (without end-point dilution) of the FFA described above.
Olfactometer bioassays
Specific attraction of Ae. malayensis to human odor was evaluated using a dual-port olfactometer (160 ×60 ×43 cm) in a controlled environment room at the London School of Hygiene and Tropical Medicine (Fig. 4A). The experiments included Ae. aegypti as an anthropophilic control. To mimic human scent, a sheer polyamide stocking washed in 70% ethanol was worn by the experimenter for 12 hours and stored at −20 °C until use. An unworn stocking, which had been cleaned in the same manner, was used as control. Prior to the experiment, 5- to 7-day-old mosquitoes were deprived of sucrose for 24 hours. Batches of 20 to 30 females were transferred into a release chamber and allowed to acclimatize for 1 hour. Stockings were thawed during 1 hour and placed inside the traps according to two distinct experimental designs. The first design, denoted “CO2 + Human odor vs. CO2 only” hereafter, consisted of one trap with human odor and one trap with no odor. The second design, denoted “CO2 only vs. CO2 only” hereafter, consisted of both traps without human odor and was used as a negative control. This negative control ensures that the device is not intrinsically biased and that mosquitoes are not preferentially attracted to one of the traps in the presence of CO2 only, a known mosquito attractant. The air stream was heated and humidified, then subsequently directed to traverse either trap until it reached the release chamber. The air stream was supplemented with CO2 (5%) released at the entrance of each trap at a rate of 175 mL/min. The air speed at the exit of the traps was set at 0.2 m/sec with a variation of no more than 0.01 m/sec between each trap (Fig. 4B,C). The sliding door of the release chamber was opened, and mosquitoes were allowed to enter the flight chamber for 20 min. During each run, mosquitoes that flew upwind towards the odor were caught inside the traps. A total of 9 replicates were performed for each mosquito species to test their attraction to human odor (CO2 + Human odor vs. CO2 only), in addition to 6 replicate runs for each species to control for any bias in the olfactometer (CO2 only vs. CO2 only). We used a Latin square design to randomize the position of each trap between replicate runs by systematically switching traps between the right and left openings. Flight activity was measured as the percentage of mosquitoes that exited the release chamber after 20 min. Attraction to human odor was estimated as the percentage of trapped mosquitoes that chose the trap with human odor over the trap with no odor in the CO2 + Human odor vs. CO2 only design. Our measure of attraction to human odor ranged from 0% (full attraction to CO2 without human odor) to 100% (full attraction to CO2 with human odor) with 50% indicating a lack of either attraction.
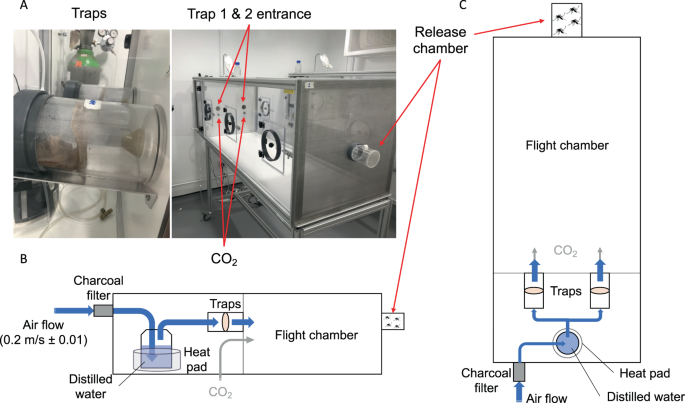
Dual-port olfactometer apparatus. (A) Pictures and (B,C) schematics (B: side view; C: top view) of the experimental setup to measure attraction to human odor.
Statistical analyses
All statistical analyses were performed using the R software version 3.5.241 and graphical representations were generated with the R package ggplot242. Vector competence was evaluated using four conventional indices. Infection rate (IR) was estimated as the proportion of blood-fed females that became infected. Dissemination rate (DR) was estimated as the proportion of infected females that developed a systemic infection (i.e., with an infected head). Transmission rate (TR) was estimated as the proportion of females with a disseminated infection that released virus in their saliva. Transmission efficiency (TE) is a summary metric estimated as the overall proportion of blood-fed females with virus-positive saliva. Vector competence indices (IR, DR, TR and TE) were analyzed with a logistic regression model in which each individual mosquito was associated with a binary variable (infected = 1, uninfected = 0), followed by an analysis of deviance with the R package car43. For DENV experiments, the initial statistical model included the species, the experiment and their interaction as explanatory variables. The experiment effect between the two DENV experiments was statistically non-significant overall and subsequently removed from the model. In the olfactometer bioassays, flight activity and attraction to human odor were analyzed using a logistic regression model where each individual mosquito was associated with a binary variable (flight activity: active = 1, inactive = 0; attraction: trap 1 = 0, trap 2 = 1), followed by an analysis of deviance with the R package car43. Both analyses accounted for the replicate run effect, which was statistically non-significant overall. Multiple comparison between conditions was performed using the estimated marginal means followed by Tukey’s post-hoc test using the R package emmeans44.
Source: Ecology - nature.com


