Physicochemical characteristics of LD slag
The major elements of the Linz-Donawitz converter slag (LD slag) were Ca, Fe, Al, Si and Mn, while the minor elements were As, Ba, Cd, Cr, Pb, Se, Ag, Sb, Ni, Zn, and Co (Supplementary Table S1). The ASTM (American Standards for Testing Materials) water leachate and the TCLP (Toxicity Characteristic Leaching Procedure) leachate of most of the heavy metals in the LD slag were very low or negligible (Table S1). The comparison of TCLP leachate concentrations of the LD slag with that of TCLP criteria recommends that the LD slag did not exceed U.S. EPA standards for considering whether the slag is environmentally hazardous. The low ASTM concentrations of metals in the LD slag further suggest that the leachate from the slag is less likely to affect groundwater above drinking water standards11,24. Whereas, the low TCLP value in the LD slag indicates that the extracted concentrations of metals from the slag in acidic conditions of intestine are negligible when ingested incidentally24.
Soil and plant properties and the crop yield
The LD slag amendment markedly increased soil organic carbon (SOC) by 11.6 and 14.6%, readily mineralizable carbon (RMC) by 37.3 and 42.7%, microbial biomass carbon (MBC) by 26.2 and 30.3%, available phosphorous (AP) by 33.2 and 33.0%, water soluble Si (aqSi) by 898 and 718%, water soluble Fe (aqFe) by 160 and 183%, exchangeable Ca2+ by 47.3 and 46.9%, exchangeable Mg2+ by 60.2 and 65.0%, whereas decreased ninhydrin reactive nitrogen (NRN) by 15.4% and 16.8% in Japonica and Indica cultivar, respectively. In addition, it increased the photosynthetic rate by 21.1 and 18.0%, straw N, P, and Si content by 20.1 and 22.2%, 17.0 and 18.4%, and 29.9 and 30.5%, grain yield by 15.2 and 13.6%, straw biomass by 19.9 and 22.0%, and root biomass by 17.2 and 19.4%, in Japonica and Indica rice cultivar, respectively (Table 1).
The nutrient uptake calculated from the dataset, although partial, magnifies the contrasts between treatments: (i) large increase of nutrient uptake with slag, (ii) large difference in nutrient uptake between Indica and Japonica species, even without slag amendment, (iii) the uptake of K is more contrasted among rice species than with slag, Si uptake is more contrasted with slag than with rice species, while the rice species and slag effects are similar for N and P (Table 2).
Soil enzyme activities
Labile carbon degrading enzymes such as α-glucosidase, β-glucosidase, α-galactosidase, β-galactosidase, α-mannosidase, and α-fucosidase which can degrade maltose, cellobiose, melibiose, lactose, mannose, and fructose, respectively, markedly increased in the slag amendment treatments in comparison to the unamended treatments, irrespective of rice cultivars (Fig. 1). Likewise, recalcitrant carbon degrading enzymes such as esterase, lipase, and N-acetyl-β-glucosaminidase which can degrade hemicelluloses and polysaccharide respectively, increased by the slag amendment. Notably, the increase was more in labile carbon degrading enzymes (mostly β-glucosidase, and β-galactosidase) compared to the recalcitrant carbon degrading enzymes. The LD slag amendment also noticeably increased soil enzyme activities involved in nitrogen cycling (i.e., leucine-aminopeptidase and trypsin) and phosphorus cycling (i.e., alkaline phosphomonoesterase and phosphohydrolase). Notably, the increase in soil enzyme activities was more prominent in Indica in comparison to Japonica rice variety. Among the C cycling enzyme, β-glucosidase, and β-galactosidase were dominant, whereas among the N cycling enzyme, aminopeptidase and among the P cycling enzyme, alkaline phosphomonoesterase were dominant in the paddy soil (Fig. 1).
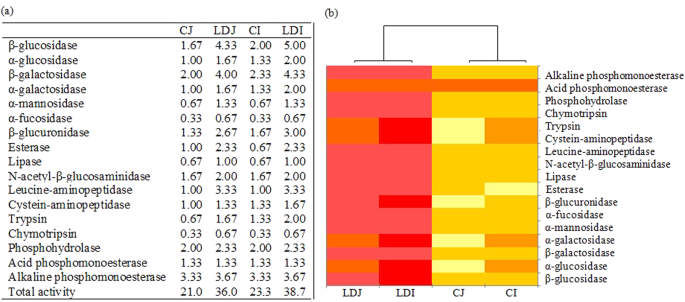
(a) Enzymatic profiles of soil based on the hydrolytic activities assessed by the APIZYM system. Values are the mean of triplicate observations, (b) Heatmap depicting significant differences in soil enzyme activities among the treatments. The relative abundance of soil enzyme activities is depicted by color intensity. The relative abundance of soil enzyme activities in different samples is colored in shades of yellow (low relative abundance) to red (high relative abundance) through orange. CJ, Japonica without slag; LDJ, Japonica with slag; CI, Indica without slag; LDI, Indica with slag.
Relative abundance and diversity of rhizosphere bacterial community
The most abundant (≥ 1.0%) bacterial phyla across treatments were Proteobacteria (34-48%), Fermicutes (15–17%), Actinobacteria (7–18%), Acidobacteria (3–7%), Bacteroidetes (2-6%), Nitrospirae (2–3%), and Chloroflexi (1–4%). The LD slag amendment significantly (P < 0.05) increased the relative abundance of Proteobacteria by 28.9 and 25.2%, and Actinobacteria by 52.2 and 50.7%, while significantly (P < 0.05) decreased the relative abundance of Acidobacteria by 31.4 and 36.1%, Bacteroidetes by 25.3 and 29.2%, Nitrospirae by 40.6 and 45.4%, and Chloroflexi by 74.7 and 61.9% in Japonica and Indica rice, respectively (Fig. 2). Within Proteobacteria, the relative abundance of Alphaproteobacteria and Betaproteobacteria significantly (P < 0.05) increased, whereas the LD slag amendment did not change Deltaproteobacteria and Gammaproteobacteria significantly, irrespective of rice cultivars (Fig. 2). The cultivar, and interaction of LD slag and cultivar did not significantly affect the relative abundance of dominant bacterial phyla (Table S2).
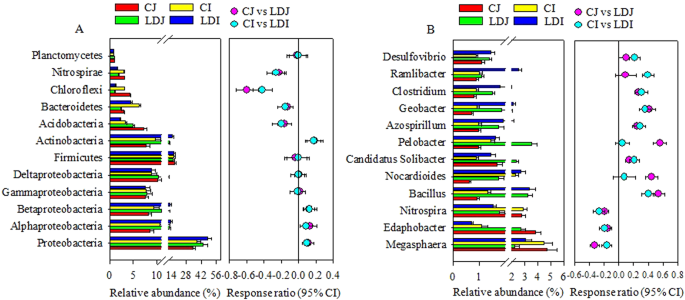
The relative abundance of major (≥1.0%) phylogenetic groups (a) and genera (b) in the rice rhizosphere as influenced by the LD slag amendment. The relative abundance is presented in terms of percentage in total bacterial sequences per sample. Significantly altered phylogenetic groups and genera were presented in term of response ratio at 95% confidence interval (CI). CJ, Japonica without slag; LDJ, Japonica with slag; CI, Indica without slag; LDI, Indica with slag.
A total of 613 bacterial genera were detected in the rhizosphere of rice. The relative abundance of genera varied greatly across the treatments. The major dominant (>1.0%) genera were presented in Fig. 2. The LD slag amendment significantly (P < 0.05) increased the relative abundance of Bacillus, Candidatus Solibacter, Azospirillum, Geobacter, and Clostridium, while significantly (P < 0.05) decreased the relative abundance of Megasphaera, Edaphobacter, and Nitrospira in both the rice cultivars (Fig. 2). However, among the dominant genera Nocardioides, and Pelobacter significantly (P < 0.05) increased only in Japonica rice cultivar, whereas Ramlibacter, and Desulfovibrio significantly (P < 0.05) increased only in Indica rice cultivar by the slag amendment (Fig. 2). The interaction of the slag and cultivar did not significantly affect the relative abundance of dominant genera (Table S2).
Much dissimilarity was observed among the treatments at the species level (Supplementary material). The LD slag amendment triggered the proliferation of some dominant species which are mostly copiotrophic, and/or have a role in plant growth promoting activities (e.g., N2 fixation, phytohormone production), iron reduction, and versatility with respect to carbohydrate utilization (Table S3). Whereas among the dominant species, species like Nitrospira moscoviensis, Candidatus Scalindua brodae, and Thauera linaloolentis which have a potential role in nitrite-oxidation, anaerobic ammonium oxidation (anammox), and denitrification, respectively were markedly decreased due to the LD slag amendment (Table S3).
The LD slag application significantly (P < 0.05) increased the Margalef’s richness and Shannon Diversity indices, but significantly (P < 0.05) decreased the Pielou’s evenness in both rice cultivars. However, no significant variation of the measured diversity indices within the cultivars was found (Table 3).
The predictor variables of rhizosphere bacterial community
Correlation analyses demonstrated that the soil pH, SOC, RMC, MBC, NRN, AP, aqSi, aqFe, photosynthetic rate, above and belowground biomass, and N, P and Si uptake (straw only) were significantly correlated with rhizosphere bacterial communities (Table 4). The CCA analysis depicted that the rhizosphere bacterial communities of slag amendment and without amendment treatments were distantly grouped (Fig. 3). The phyla Alphaproteobacteria, Betaproteobacteria, Actinobacteria, and the genera Bacillus, Pelobacter, Azospirillum, Geobacter, Clostridium, Ramlibacter, and Desulfovibrio were significantly and positively correlated with SOC, RMC, MBC, aqSi, photosynthetic rate, aqFe, AP, straw Si, N, and P uptake, and above and belowground biomass, whereas the phyla Nitrospirae, Chloroflexi and the genera Megasphaera and Nitrospira were significantly and positively correlated with NRN (Fig. 3, Table S4). The VPA analysis revealed that a set of soil nutrient attributes (i.e., SOC, RMC, NRN, AP, aqSi, and aqFe), plant attributes (i.e., photosynthesis, straw Si, N, and P uptake, and above- and belowground biomass), and soil pH accounted for 39.7, 28.9, and 5.6% of rhizosphere bacterial community variations, while 25.6% of the variation was unexplained (Fig. 4). Notably, nutrient status, plant attributes and soil pH had significant contribution to the variation in the rhizosphere bacterial community (Fig. 4).
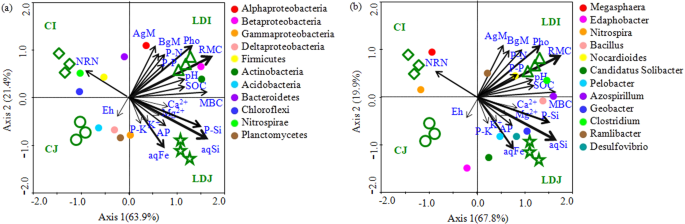
Canonical correspondence analysis (CCA) relating selected soil and plant variables to major phylogenetic groups (a) and genera (b). The resulting ordination biplot approximated the weighted average of each group/taxa with regard to each of the measured variables, which are represented as arrows. The lengths of these arrows indicate the relative importance of measured variables, whereas the angle between the arrows and the axis reflects the degree to which they are correlated. To statistically evaluate the significance (P < 0.01) of the first canonical axis and of all canonical axes together, the Monte Carlo permutation full model test with 999 unrestricted permutations was performed. MBC, Microbial biomass C; SOC, soil organic C; RMC, readily mineralizable C; NRN, ninhydrin nitrogen content; AP, available P, aq, water soluble; Pho, photosynthesis; AgM, Aboveground biomass; BgM, Belowground biomass; and P-N, P-P, P-K, and P-Si are straw N, P, K, and Si uptake, respectively.
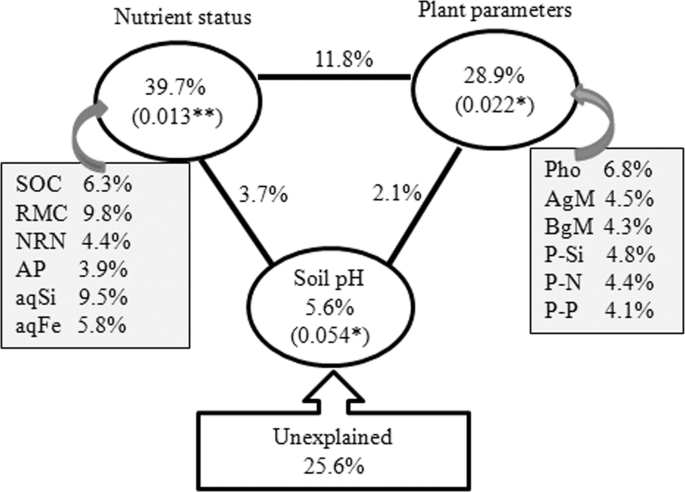
Variation partitioning analysis (VPA) of rhizosphere bacterial communities among important plant parameters, soil nutrient status, soil pH, and their interactions. The values in parentheses are P-values. Abbreviations for the measured variables are provided in Fig. 3.
Source: Ecology - nature.com


