Champsosaurus brain endocast
CMN 8920 (Champsosaurus lindoei) was scanned at a higher resolution than CMN 8919 (C. natator), and therefore more accurately illustrates details of the endocranial anatomy of Champsosaurus. As such, CMN 8920 forms the basis of this description, and only notable differences with CMN 8919 will be discussed. Taphonomic deformation of both braincases is minimal, so we infer that the reconstructed endocasts accurately reflect the living brain endocast morphology.
The brain endocast of CMN 8920 is narrow both mediolaterally and dorsoventrally, and does not show flexure (Fig. 2). In contrast, the brain endocast of CMN 8919 shows distinct cerebral and pontine flexures, an observation that is corroborated by fragmentary specimens of C. natator (ROM 688). The walls around the midbrain and hindbrain are well-ossified and provide good anatomical detail, but the lateral and ventral walls around the olfactory stalks did not ossify, and the exact shape of the olfactory stalks therefore cannot be determined. The ossified braincase of CMN 8920 is approximatley 32 mm long, but impressions of the olfactory stalks of the brain on the ventral surface of the parietals and frontals show that the entire brain cavity is 67 mm long (Fig. 3). The olfactory stalks are substantial (approximately 37 mm long from the anterior margin of the pineal body) and occupied approximately 55% of the total length of the brain endocast.
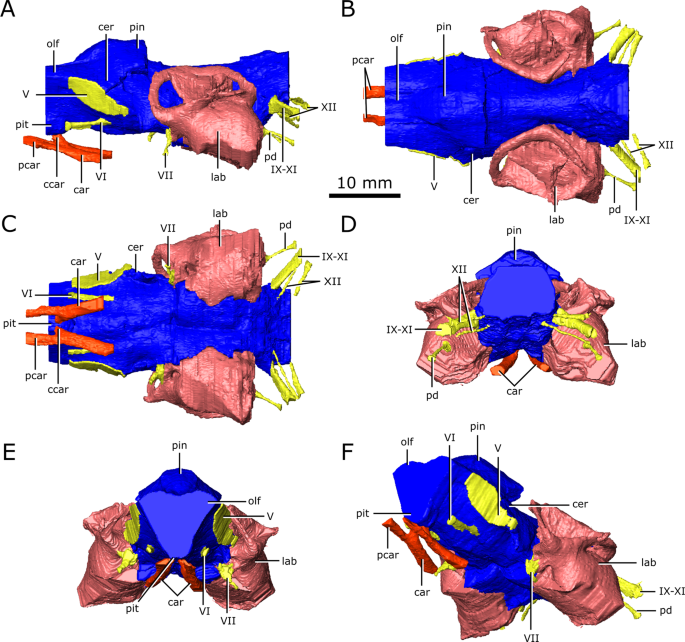
Reconstruction of the endocranial anatomy of Champsosaurus lindoei (CMN 8920). (A) left lateral view; (B) dorsal view; (C) ventral view; (D) posterior view; (E) anterior view; (F) left anterolateroventral view. The brain endocast is illustrated in blue, endosseous labyrinth in pink, cranial nerves in yellow, and carotid artery in red. Abbreviations: car, carotid arteries; ccar, cerebral branch of the carotid arteries; cer, cerebrum; lab, endosseous labyrinth; olf, base of the olfactory lobes; pcar, palatine branch of the carotid arteries; pd, canal for the parilymphatic duct; pin, pineal body; pit, pituitary fossa; IX-XI, canal for cranial nerves IX, X, and XI; XII, canal for cranial nerve XII; V, opening for cranial nerve V; VI, canal for cranial nerve VI; VII, canal for cranial nerve VII. Images generated in Amira 5.4.3 (https://www.fei.com/software/amira/) and processed in Inkscape 0.92 (https://inkscape.org/).
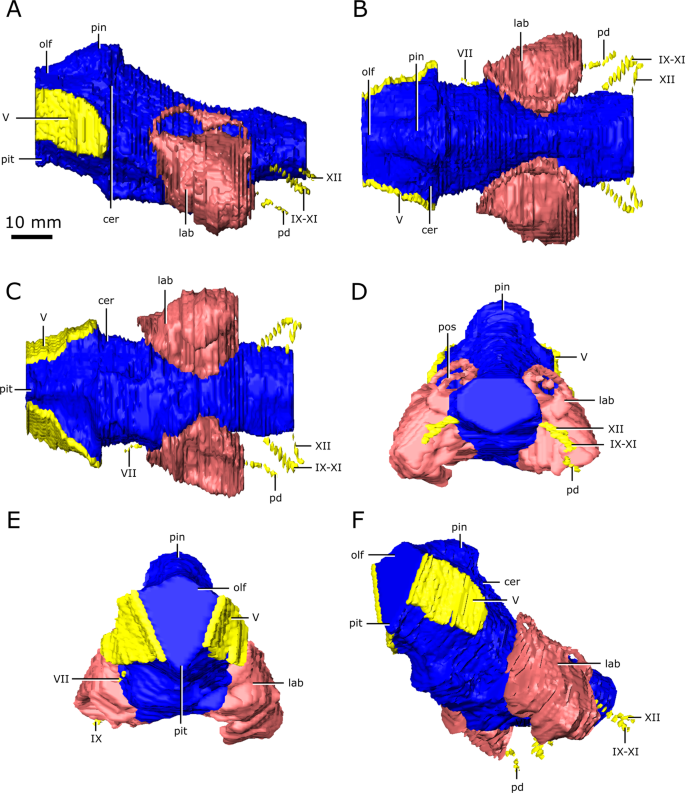
Reconstruction of the endocranial anatomy of Champsosaurus natator (CMN 8919). (A) left lateral view; (B) dorsal view; (C) ventral view; (D) posterior view; (E) anterior view; (F) left anterolateroventral view. The brain endocast is illustrated in blue, endosseous labyrinth in pink, cranial nerves in yellow, and carotid artery in red. Abbreviations: cer, cerebrum; lab, endosseous labyrinth; olf, base of the olfactory lobes; pd, canal for the parilymphatic duct; pin, pineal body; pit, pituitary fossa; IX-XI, canal for cranial nerves IX, X, and XI; XII, canal for cranial nerve XII; V, opening for cranial nerve V; VII, canal for cranial nerve VII. Images generated in Amira 5.4.3 (https://www.fei.com/software/amira/) and processed in Inkscape 0.92 (https://inkscape.org/).
The ossified braincase of CMN 8919 is approximately 66 mm long and the entire brain cavity is approximately 126 mm long (Fig. 3). The olfactory stalks of the brain of CMN 8919 are also elongate, measuring approximatley 65 mm long from the anterior margin of the pineal body, and occupy approximatley 51% of the length of the brain endocast. Two foramina lead into the ventral surface of the frontals in the impression left by the anterior-most extent of the olfactory stalks of CMN 8920. CT data reveal that these foramina fork and dissipate into the cortical bone of the frontals, suggesting that they are vascular and carried diploic veins (Fig. 4; dv)4.
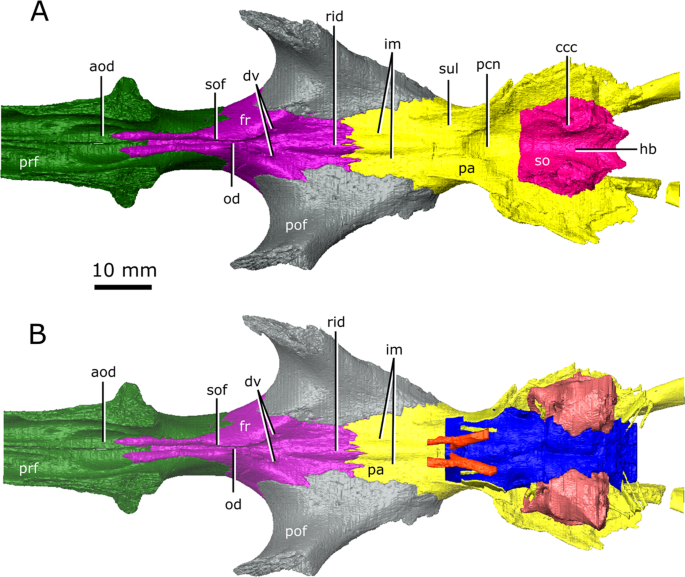
The isolated braincase roof (prefrontals, frontals, postfrontals, parietals, supraoccipital) of CMN 8920. (A) Ventral view of the isolated braincase roof; (B) Ventral view of the braincase roof with the segmented brain endocast (blue), endosseous labyrinth (pink), cranial nerves (yellow), and carotid arteries (red) in position. The braincase roof is slightly faded. Abbreviations: aod, anterior of olfactory duct; ccc, canal for the crus communis; dv, diploic vein foramen; fr, frontal; hb, roof of the hindbrain; im, impressions of the olfactory tracks; od, olfactory duct; pcn, parietal concavity for the pineal body; pa, parietal; pof, postfrontal; prf, prefrontal; rid, ridge seperating the paired olfactory tracts; so, supraoccipital; sof, subolfactory flange; sul, area inundated with sulci. Images generated in Amira 5.4.3 (https://www.fei.com/software/amira/) and processed in Inkscape 0.92 (https://inkscape.org/).
The brain endocasts of both specimens are fully enclosed by bone posterior to the olfactory stalks, preserving the morphology of the midbrain and hindbrain. The lateral, posterior, and ventral walls of the pituitary fossa are formed by the basisphenoid, but the anterior wall did not ossify. The pituitary fossa of both specimens is shallow, wide, and lacking details such as sulci, suggesting the pituitary gland would not have occupied the entirety of this space, and was supported by a thick layer of dura matter.
Posterior to the pituitary fossa, the endocast expands dorsally into a large concavity in the ventral surface of the parietals (Fig. 2; Fig. 3; pin). Russell17 interpreted this expanson as a portion of the cerebellum, but based on its position in the midbrain, it appears to be the pineal expansion that is also present in other neodiapsids (e.g., phytosaurs53). The CT data of CMN 8920 show that the dorsal surface of the brain endocast anterior to the pineal expansion possesses sulci, suggesting that the brain pressed close to the skull in this region (Fig. 4; sul). The CT data of CMN 8919 are of too low resolution to capture these fine details; however, these sulci are also present on fragmentary specimens of C. natator (CMN 8921; CMN 8922; CMN 32579; ROM 688), suggesting that sulci in this region are widely present in Champsosaurus.
Posterior to the pineal expansion, the brain endocast narrows both dorsoventrally and mediolaterally, and is flanked on either side by the endosseous labyrinths (Fig. 2; Fig. 3; lab). The optic lobes, cerebellum, and flocculus are not evident in the brain endocast of either CMN 8920 or CMN 8919, and were likely small and covered by thick dura matter. The parasphenoid forms the floor of the braincase medial to the endosseous labyrinths (Fig. 5). A strong sagital keel is present on the dorsal surface of the parasphenoid and posterior-most portion of the basisphenoid that axially bisects the ventral portion of the endocranial cavity that housed the brain stem (Fig. 5; k). The keel is evident in the CT data for CMN 8920 and is also present in fragmentary specimens (CMN 8922; ROM 688), but it is not seen in the CT data for CMN 8919, likely due to a combination of low scan resolution and damage during preperation. Fox25 described this region of the endocast as a deep basin, but did not comment on the presence of a dorsal keel on the parasphenoid. Posterior to the parasphenoid, the brain endocast is floored by the basioccipital and expands dorsally and mediolaterally, but does not reach the same width or height as it does anterior to the auditory system. The brain endocast extends posteriorly and opens to the foramen magnum.
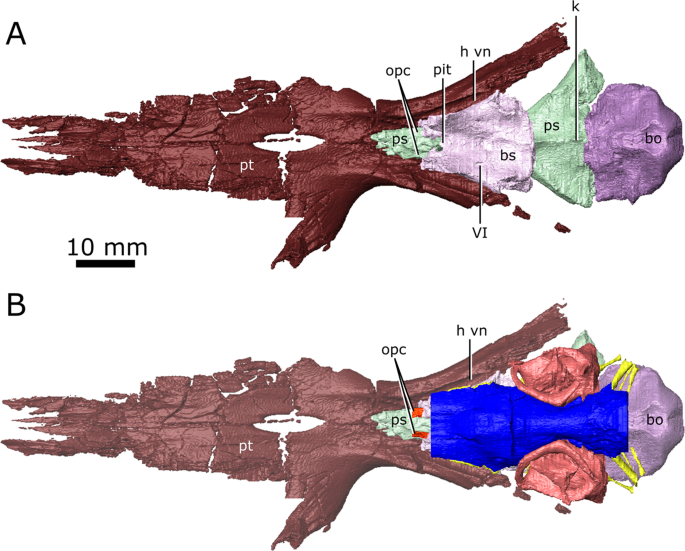
The isolated braincase floor (pterygoids, basisphenoid, parasphenoid, and basioccipital) of CMN 8920. (A) Dorsal view of the isolated braincase floor; (B) Dorsal view of the braincase floor with the segmented brain endocast (blue), endosseous labyrinth (pink), cranial nerves (yellow), and carotid arteries (red) in position. The braincase floor is slightly faded. Abbreviations: bo, basioccipital; bs, basisphenoid; hvn, trough for the lateral head vein; k, parasphenoid keel; opc, opening for the palatine branch of the carotid artieries; pit, pituitary fossa; ps, parasphenoid; pt, pterygoid; VI, exit for cranial nerve VI. Images generated in Amira 5.4.3 (https://www.fei.com/software/amira/) and processed in Inkscape 0.92 (https://inkscape.org/).
Cranial nerves
The cranial nerve passages of CMN 8919 could not be observed due to low scanning resolution, but were clearly visible in CMN 8920. The following description is based predominantly on the cranial nerve passages of CMN 8920 (Fig. 6).
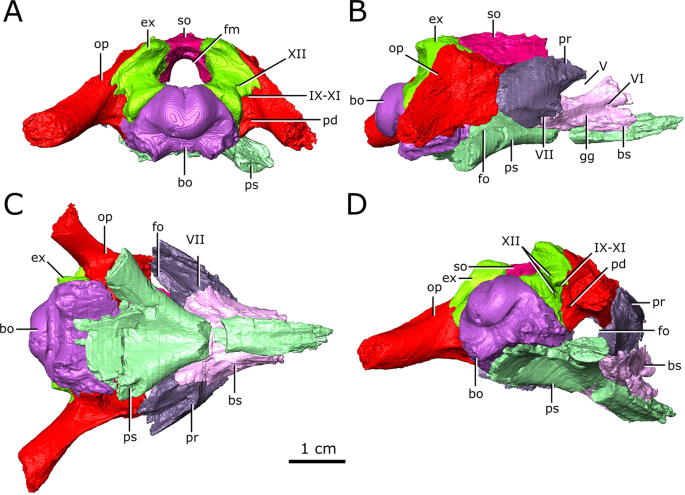
Isolated chondrocranial elements and parasphenoid of CMN 8920. (A) posterior view; (B) right lateral view; (C) ventral view; (D) posterolateroventral view. Abbreviations: bo, basioccipital; bs basisphenoid; ex, exoccipital; fm, foramen magnum; fo, fenestra ovalis; gg, depression for the gasserian ganglion; op, opisthotic; pd, foramen for the perilymphatic duct; pr, prootic; ps, parasphenoid; so, supraoccipital; Roman numerals indicate the foramina of the corresponding cranial nerves. Images generated in Amira 5.4.3 (https://www.fei.com/software/amira/) and processed in Inkscape 0.92 (https://inkscape.org/).
The olfactory duct is preserved between the subolfactory flanges (= crista cranii of some other diapsid groups, such as lepidosaurs54) on the ventral surface of the frontals, extending from the posteriormost portion of the olfactory chambers of the nasal passages to the region occupied by the olfactory bulbs of the brain (Fig. 4; od). As mentioned previously, the anterior braincase did not ossify in Champsosaurus, and the pathways for cranial nerves II-IV are not preserved. Dorsal to the pituitary fossa, there is a large, paired opening in the walls of the braincase that is bordered by the parietal dorsally, and the basisphenoid ventrally (Fig. 6; V). This opening likely carried the trigeminal nerve (CN V) as it exited the endocast, but does not show evidence for the divergence of CN V into its three rami, CN V1 (ophthalmic), CN V2 (maxillary), and CN V3 (mandibular), suggesting that this divergence would have occurred outside of the boney braincase. Supporting the extra-cavity split of CN V is a shallow depression in the lateral wall of the basisphenoid ventral to the opening for CN V that may have held the gasserian ganglion (Fig. 6; gg). A shallow groove extends posteriorly from the opening for CN V along the lateral surface of the parietal and neomorph towards the pterygoquadrate foramen that may represent the path of CN V3, although it is also possible that the groove represents the path of the stapedial artery (see Vasculature section below for discussion). The only preserved portion of the pathway for CN V2 is in the snout, originating in the ventral rim of the orbit (see Supplementary Figure S3) and extending anteriorly through the maxilla and premaxilla to the tip of the snout. The canal branches repeatedly along its length, where the branches lead to the outer surface of the skull and likely carried sensory nerves to innervate the snout. CN V1 would have extended anterodorsally to inervate the orbit and integument of the snout55, but was supported by soft tissue and its pathway was not preserved.
Posterior to the pituitary fossa, a paired canal, which likely carried the abducens nerve (CN VI), extends anteriorly from the floor of the brain cavity and exits through the lateral surface of the basisphenoid (Fig. 6; VI). A paired canal, which likely carried the facial nerve (CN VII), extends ventrolaterally from the floor of the endocast anterior to the labyrinth (Fig. 6; VII). The left CN VII canal passes between the basisphenoid and the prootic for its entire length, but the right nerve canal exits directly through the prootic, only contacting the basisphenoid for a small portion anteriorly near the ventral surface of the skull. There is no osseous canal for the vestibulocochlear nerve (CN VIII). Instead, the otic capsule communes to the brain cavity through a broad opening, suggesting that the canal for CN VIII and the majority of the medial wall of the otic capsule were cartilagenous in life. The absence of an osseous canal for CN VIII in CMN 8920, CMN 8919, and other specimens of Champsosaurus25 suggests that a cartilagenous medial wall to the otic capsule was widely present in Champsosaurus.
A canal originates at the posterior wall of the otic capsule and extends posteriorly through the opisthotic. We previously interpreted this as the passage for the glossopharyngeal nerve25,56 (CN IX). More likely, this canal carried the perilymphatic duct (Fig. 2; Fig. 3; pd) extending posteriorly from the otic capsule57, and CN IX would instead have exited with the vagus (CN X) and accessory (CN XI) nerves, as seen in some modern reptiles such as crocodilians1. A canal extends ventrolaterally from the endocast and exits between the opisthotics and exoccipitals (Fig. 2; Fig. 3; Fig. 6; IX-XI). The relatively large diameter, and the position of the canal between the opithotic and exoccipital, suggests that it is the vagal foramen and would have carried CN IX, CN X, and CN XI57,58,59. Posterior to the canal for CN IX, CN X, and XI, two paired, narrow canals that carried branches of hypoglossal nerve (CN XII) exit ventromedially at the opening for the foramen magnum and extend posterolaterally through the exoccipitals (Fig. 2; Fig. 3; Fig. 6; XII).
Endosseous labyrinth
Dorsally, the anterior and posterior semicircular canals appear as distinct, tubular structures (Fig. 7; asc; psc); however, the entire lateral canal of CMN 8919 is confluent with the dorsolateral surface of the pars inferior, as is the anterior half of the lateral canal of CMN 8920 (Fig. 7; lsc). This suggests that the medial wall of the lateral semicircular canal was poorly ossified in Champsosaurus, and that regions of the labyrinth, such as the lateral canal, would have been supported by soft tissue and cartilage within the otic capsule in life. Additionally, the prootic, opisthotic, and supraoccipital fail to contact each other lateral to the labyrinth in CMN 8920, creating a cavity that projects dorsolaterally from the pars inferior (Fig. 7; gp). This cavity is absent in CMN 8919, suggesting that the otic region was better ossified in larger animals, although the lateral canal is no better preserved in CMN 8919 than it is in CMN 8920. The angle of the lateral canal from the long axis of the skull varies considerably between CMN 8920 and CMN 8919, where the lateral canal is oriented approximately −15.8° from the long axis of the skull in CMN 8920 (tilted anteroventrally), and approximately 13.3° from the long axis of the skull in CMN 8919 (tilted anterodorsally).
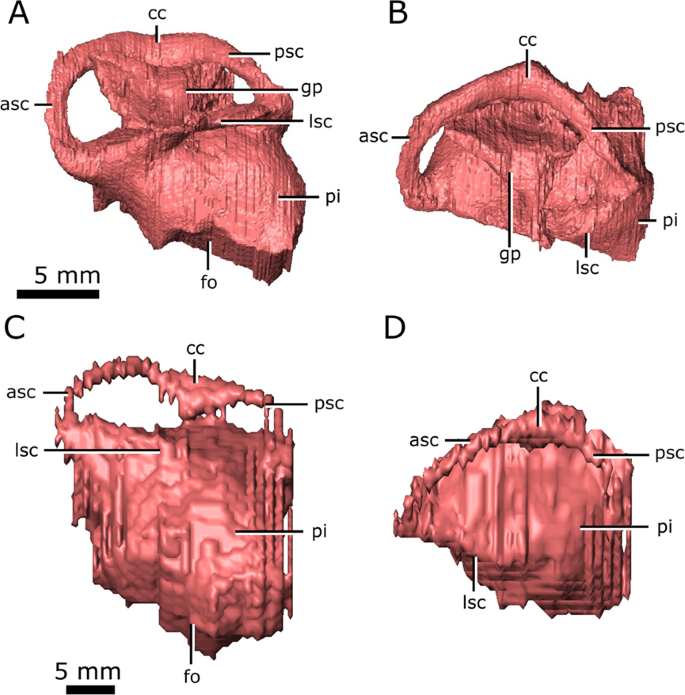
Left endosseous labyrinth of Champsosaurus lindoei (CMN 8920) in: (A) lateral view; and (B) dorsal view, and Champsosaurus natator (CMN 8919) in: (C) lateral view; and (D) dorsal view. Abbreviations: asc, anterior semicircular canal; cc, crus communis, fo, fenestra ovalis; gp, unossified gap between the prootic, opisthotic, and supraoccipital; lsc, lateral semicircular canal; pi, pars inferior; psc, posterior semicircular canal. Images generated in Amira 5.4.3 (https://www.fei.com/software/amira/) and processed in Inkscape 0.92 (https://inkscape.org/).
The anterior ampullae are small, but can be distinguished as an enlargement of the canals at their anteriormost extent. The posterior ampulla is not evident in the endosseous labyrinth, but would have been located at the posteriormost extent of the posterior canal. In CMN 8920 and CMN 8919 the pars inferior forms a bulbous cavity ventral to the semicircular canals. The fenestra ovalis is located on the ventral surface of the pars inferior (Fig. 7; fo), with no portion of the endosseous labyrinth extending ventral to it (e.g., the cochlear duct), possibly due to the dorsoventrally flattened skull profile of Champsosaurus. This is in contrast to the morphology of most reptiles, where the fenestra ovalis is located on the lateral surface of the pars inferior and the cochlear duct extends ventral to it. There is no clear separation between the cochlear duct and the sacculus in CMN 8920 and CMN 8919.
Vasculature
The passages for the internal carotids are not visible in the CT data for CMN 8919, and the morphology of these arteries is based entirely on CMN 8920. The internal carotids entered the skull through passages on the ventral surface of the skull that passed between the contact of the parasphenoid and pterygoid, and extended anterodorsally towards the pituitary fossa (Fig. 2; car). Ventral to the pituitary fossa, the canals fork, where the dorsal branch carried the cerebral artery that opened into the pituitary fossa (Fig. 2; ccar), and the ventral branch carried the palatine artery, continuing anteriorly until it opens on the dorsal surface of the pterygoid, anterior to the basisphenoid (Fig. 2; pcar). The path of the palatine artery canals anterior to the basisphenoid cannot be determined due to incomplete ossification of this region.
The lateral head vein sits in a deep trough formed by the quadrate ramus of the pterygoid, and the lateral wall of the basisphenoid (Fig. 5; h vn). Fox25 stated that a channel is imprinted on the lateral wall of the clinoid process of the basisphenoid that drained the orbital sinus into the lateral head vein, but this channel is not present in CMN 8920 and CMN 8919. Additionally, Fox25 described a foramen penetrating the quadrate ramus of the pterygoid that carried the lateral head vein, but no such foramen is seen in either CMN 8920 or CMN 8919. Instead, the lateral head vein appears to have extended posteriorly along the trough formed by the pterygoid and basisphenoid, and exited the skull lateral to the fenestrae ovales. Fox25 also suggested that a groove imprinted into the lateral surface of the parietal and neomorph leading from the exit for CN V to the pterygoquadrate foramen is an impression of the stapedial artery. If Fox’s25 interpretation is correct, the stapedial artery would have divided into the superior and inferior branches anterior to the opening for CN V25.
Nasal cavity
Like the snout of CMN 8920, the nasal passage is highly elongate, measuring approximately 14 cm from the anteriormost extent of the narial opening to the posteriormost extent of the olfactory chambers (Fig. 8). An ossified internarial septum is absent, but longitudinal ridges at the confluence of the left and right vomers, and the midline of the internarial and nasal, suggest it would have been present as cartilage in life.
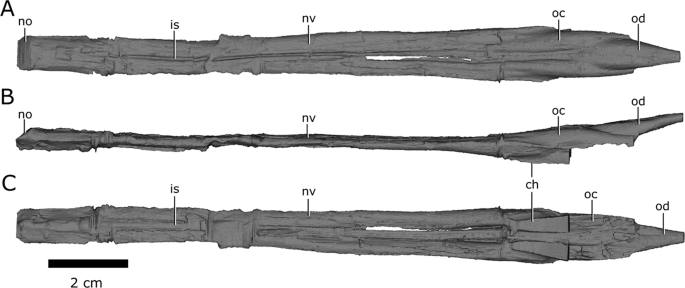
Left and right nasal passages of Champsosaurus lindoei (CMN 8920), in (A) dorsal view; (B) left lateral view; and (C) ventral view. Abbreviations: ch, choana; is, ridge indicating the internarial septum; no, narial opening; nv, nasal vestibule; oc, olfactory chamber; od, olfactory duct. Images generated in Amira 5.4.3 (https://www.fei.com/software/amira/) and processed in Inkscape 0.92 (https://inkscape.org/).
The nasal passages are ovoid in cross-section, each measuring approximately 0.8 cm in width, and 0.45 cm in height. The floor of the nasal passage is severely damaged, and several fragments of bone have been displaced dorsally into the nasal passage. The fragmentation is most prominent midway along the nasal passage, and along the posteriormost extent of the olfactory chambers. The dorsolateral walls of the olfactory chambers are well-preserved, revealing that the olfactory chambers are also elongate, measuring approximately 2.9 cm in length (Fig. 8; oc). The choanae open ventrally from the anterior floor of the olfactory chambers between the palatine laterally and the vomer medially (Fig. 8; ch).
The single olfactory duct extends posterodorsally from the olfactory chambers towards the brain endocast, between the paired subolfactory flanges of the frontals (Fig. 4; Fig. 8; od), and narrows posteriorly to form the passage for the olfactory nerve (CN I). With the exception of fragmentation of the floor of the nasal passage, the osseous walls of the nasal passage are smooth, and there are no ridges present that are suggestive of turbinates.
Auditory capabilities
The mean best hearing frequency and best hearing range were plotted against the endocochlear duct length for the extant taxa from the data provided by Walsh et al.7. The length of the scaled and transformed pars inferior for CMN 8920 (−0.65698) was inserted into the derived equations of the regression lines (Fig. 9), resulting in a best hearing frequency of 1798.8 Hz, and a best hearing range of 2936.5 Hz (overall best hearing range: 330.6–3267.1 Hz).
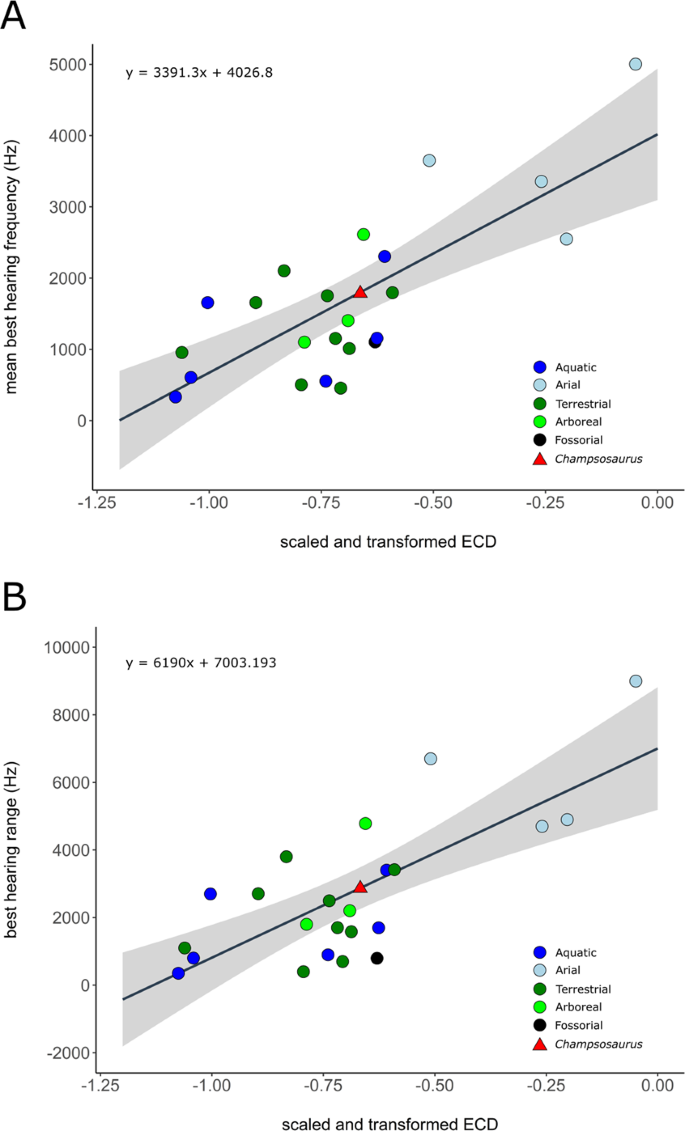
Correlation between scaled and transformed endocochlear duct length (ECD) and (A) mean best hearing frequency (y = 3391.3x + 4026.8; r2 = 0.5825; p = 2.28e-05); (B) best hearing range (y = 6190x + 7003.193; r2 = 0.5521; p = 4.875e-05) with ecologies coloured separately. Grey area indicates the 95% confidence interval of the regression line. Extant data from Walsh et al. (2009). Red triangle indicates the predicted value for Champsosaurus lindoei (CMN 8920).
Geometric morphometrics of the semicircular canals
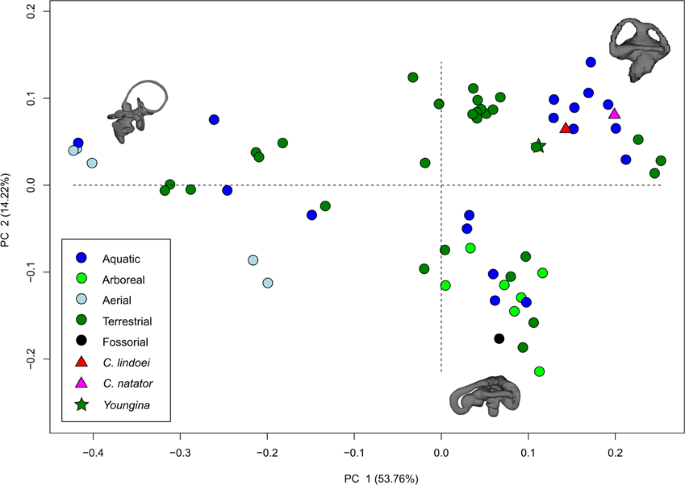
The PCA produced 62 PC axes, but only axes 1 through 3 will be discussed here (cumulative variation = 74.78%) because the remaining PC axes account for relatively little variation (<5% each). Plotting PC1 against PC2 (Fig. 10) shows that PC1 (53.76% of variation) mostly represents curvature of the anterior semicircular canal, angling of the posterior canal relative to the rest of the labyrinth, curvature of the lateral canal, and angling of the lateral canal relative to the rest of the labyrinth. Positive PC1 values represent a dorsoventrally compressed, less curved anterior canal, a posterodorsally angled posterior canal, and an anterodosally angled lateral canal that is less curved anteriorly. Negative PC2 values represent a dorsoventrally elongated and curved anterior semicircular canal, an anterodorsally angled posterior canal, and an anteroventrally angled lateral canal that is more curved anteriorly. Birds, which plot towards PC1 negative, have the most extreme condition, with an anterior semicircular canal that arcs over the posterior semicircular canal and enters the crus comunis posteriorly, a posterior canal that is strongly angled anteroventrally relative to the rest of the labyrinth, and an anteroventrally angled lateral canal relative to the rest of the labyrinth. PC2 (14.22% of variation) mostly represents anteroposterior elongation and out-of-plane curvature (torsion) of the anterior semicircular canal, curvature of the posterior canal, and torsion of the lateral canal. Positive PC2 values represent anteroposterior compression and medial torsion of the anterior canal, a more tightly curved posterior canal, and less torsion in the lateral canal. Negative PC2 values represent anteroposterior elongation and lateral torsion of the anterior canal, a more widely curved posterior canal, and greater torsion of the lateral canal. PC3 (6.80% of variation; Fig. 11) represents a combination of curvature of the anterior canal, angle of the anterior and posterior canals relative to one another, and the curvature of the lateral canal. Positive PC3 values indicate a smaller, more eliptical anterior canal, a more acute angle between the anterior and posterior canals, and a more widely curved lateral canal. Negative PC3 values indicate a larger, more curved anterior canal, a more abtuse angle between the anterior and posterior canals, and tighter curvature of the lateral canal.
PC 1 vs PC 2, representing 67.98% of the total variation. Taxa are colour coded based on ecology. End-point morphologies: top right, Tomistoma schlegelii; bottom, Aheatulla nasuta; top left, Passer domesticus.
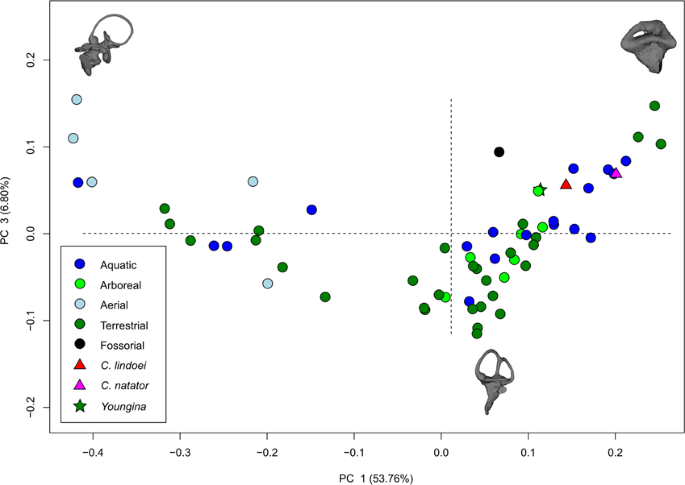
PC 1 vs PC 3 representing 60.56% of the total variation. Taxa are colour coded based on ecology. End-point morphologies: top left, Passer domesticus; bottom, Erlikosaurus andrewsi; top right, Manouria emys.
When visualizing PC1 vs PC2 (Fig. 10), three distinct groups are formed that appear separated by phylogeny: Aves (left), Lepidosauria (bottom right), and non-avian archosauromorphs (top right). Projecting a phylogeny (see Supplementary Fig. S4) onto PC1 vs PC2 (see Supplementary Fig. S5) clearly illustrates these groups, suggesting that phylogeny strongly influences grouping in this PCA.
Phylogenetic signal was less than expected under Brownian motion (K < 1; see Supplementary Table S1) in both centroid size and canal morphology. The phylogenetic signalling in both centroid size and canal morphology was statistically significant (p = 0.007, and p = 0.001, respectively), suggesting that closely related species tend to have similarly sized labyrinths and similar canal morphologies.
Both specimens of Champsosaurus plot close to the non-avian archosauromorphs and Youngina (see Discussion for phylogenetic implications). Within the non-avian archosauromorph group, the Champsosaurus specimens plot closest to the aquatic taxa (Fig. 10; e.g., crocodilians and turtles), suggesting that the semicircular canals of Champsosaurus are most similar in morphology to aquatic archosauromorphs.
ANCOVA (see Supplementary Table S2) indicates a moderate and significant relationship between ecology and canal shape (R2 = 0.25979, p = 0.0004), and a weak but significant relationship between centroid size and canal shape (R2 = 0.04334, p = 0.0045). The interaction between ecology and centroid size was found to have a weak and insignificant relationship with canal shape (R2 = 0.02470, p = 0.4539).
PGLS (see Supplementary Table S3) indicates similar relationships to the ANCOVA, with a moderate relationship between ecology and canal shape that approaches significance (R2 = 0.21451, p = 0.0173; Bonferroni corrected α-level = 0.0166), and a weak, but significant relationship between centroid size and canal shape (R2 = 0.02882, p = 0.0065). The interaction between ecology and centroid size was also found to have a moderate and significant relationship with ecology (R2 = 0.09681, p = 0.0026). It is interesting to note that the correlation coefficients for ecology and centroid size were lower in the PGLS than in the ANCOVA, and the correlation coefficient for the interaction of ecology and centroid size was higher in the PGLS than in the ANCOVA. This is most likely because there is an interaction between ecology, centroid size, and phylogeny, meaning that ecology and centroid size are not totally independent of phylogeny13. This is supported by Bloomberg’s K value for centroid size (K = 0.1793, p = 0.007), suggesting that centroid size is significantly influenced by phylogeny.
Visualization of the CVA (Fig. 12) and posterior probabilities (see Supplementary Table S4) show that the ecological groups occupy significantly different regions of morphospace (p < 0.01). The classification accuracy of the CVA was approximately 85%. CV1 is mostly separated by PC1, where positive CV1 values represent increased curvature of the anterior canal, an anterodorsally angled posterior canal, and an anteroventrally angled lateral canal with greater curvature anteriorly. Aerial taxa plot towards CV1 positive, while arboreal, aquatic, and terrestrial taxa plot towards CV1 negative. Both Champsosaurus specimens plot towards CV1 negative (Fig. 12). CV2 is mostly influenced by PC2, where positive CV2 values represent anteroposterior elongation and lateral torion of the anterior canal, a more widely curved posterior canal, and greater torsion of the lateral canal. Arboreal taxa plot towards CV2 positive, while aquatic, terrestrial, and aerial taxa plot towards CV2 negative. Champsosaurus lindoei and C. natator plot towards CV2 negative (Fig. 12). CV3 is mostly influenced by PC3, where negative CV3 values represent a smaller, more eliptical anterior canal, a more acute angle between the anterior and posterior canals, and a more widely curved lateral canal (Fig. 12). Terrestrial taxa plot towards CV3 positive, and aquatic, arboreal, and aerial taxa plot towards CV3 negative. Both Champsosaurus species plot towards CV3 negative.
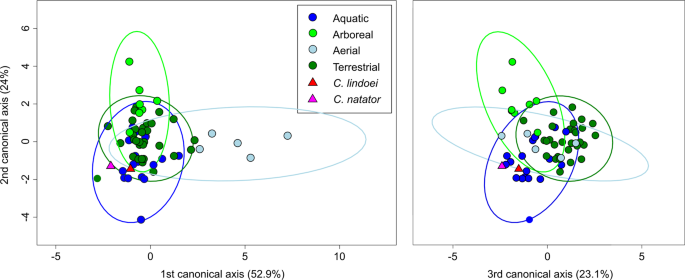
CV1 vs CV2 (left) representing 76.9% of the total between-group variation, and CV3 vs CV2 (right) representing 47.1% of the total between-group cariation. 95% confidence ellipses of each ecological group are plotted.
Posterior probabilities of Mahalanobis distances found that C. natator occupies a significantly different region of morphospace from the arboreal, aerial, and terrestrial ecological groups (p < 0.0001), but does not occupy a significantly different region from the aquatic group (see Supplementary Table S5). Similarly, C. lindoei occupies a significantly different region of morphospace from the arboreal and aerial groups (p < 0.0001), but does not occupy a significantly different region of morphospace from the aquatic or terrestrial groups. It should be noted that the difference between C. lindoei and the terrestrial group approaches significance (p = 0.019, Bonferroni corrected α-level = 0.00625), and is significant in the uncorrected pairwise comparisons. Both Champsosaurus specimens plot well within the 95% confidence ellipse for the aquatic group, and log-likelihood estimations clearly assign both Champsosaurus specimens to the aquatic group (>0.95 likelihood).
Source: Ecology - nature.com


