Hydrocarbons in deep-sea hydrothermal field samples
The concentration and composition of hydrocarbons were measured in the samples from hydrothermal fields at the southern Mid-Atlantic Ridge (SMAR), southwest Indian Ridge (SWIR), and East Pacific Rise (EPR), and in vent plumes, sulfide chimney samples, and sediments (Table S2). In the five plume samples from SMAR, the total hydrocarbon concentrations (THC) ranged from 462.6 to 742.1 μg·L−1 (Fig. 1a). In contrast, THC in the nearby non-plume deep-sea water was only 10.3 μg·L−1 (Table S2). In the chimney sulfide samples, THCs were present at concentrations of 17.1, 15.3, and 13.2 μg·g−1 (dry weight) in the SMAR, EPR, and SWIR samples, respectively (Table S2), with PAHs, n-alkanes (C14–C28), and branched alkanes (C16–C20) as the major components (Fig. 1b). In the hydrothermal sediments, the THCs ranged from 2.9 to 3.9 μg·g−1 (dry weight), with high concentrations of polycyclic aromatic hydrocarbons containing 2–5 rings detected in all of the sediment samples; however, only a few types of alkanes were detected, and these were present at low concentrations (Fig. 1c and Table S2).
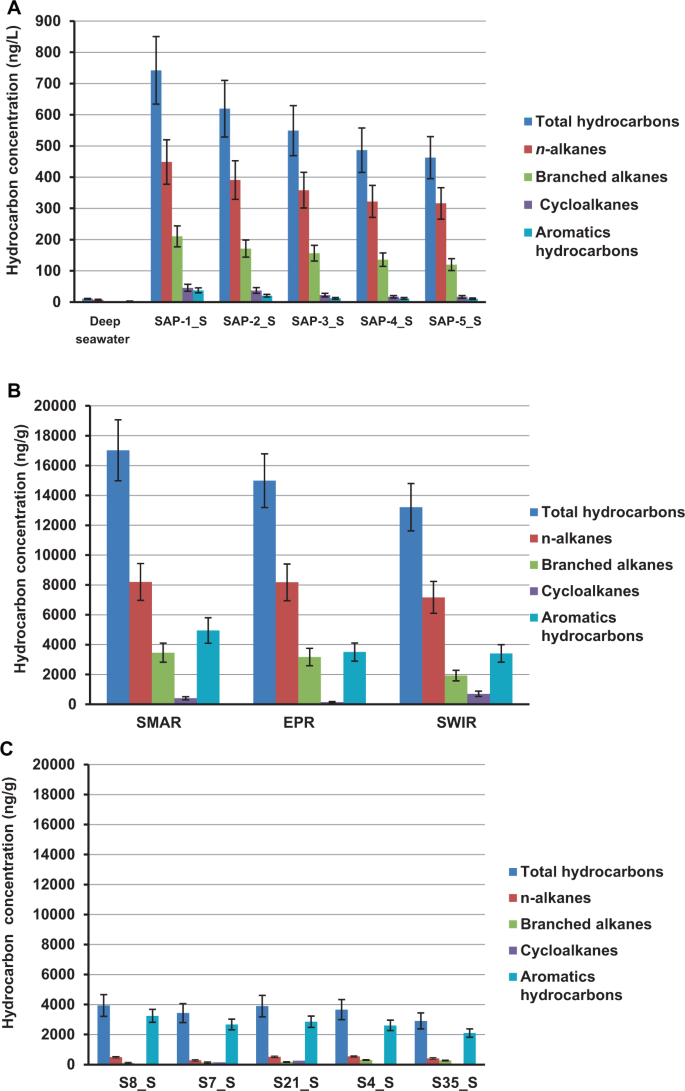
Hydrothermal vent plume samples (a); hydrothermal chimney samples (b); hydrothermal sediment samples (c). Data are the means of three independent measurements.
Bacterial diversity in the hydrothermal plume in situ
The bacterial community composition of the plume samples of the newly discovered hydrothermal field named Deyin-1 on the south MAR (15°S) is shown in Fig. 2. In the rising plume sample (SAP-1_S), the 16S rRNA gene sequences related to gamma-proteobacteria (31.5%) and epsilon-proteobacteria (19.2%) were highly abundant (Fig. 2). Among the detected gamma-proteobacteria, levels of the following genera were relatively high Alcanivorax (7.4% of the total), Glaciecola (6.7%), Marinobacter (3.7%), SUP05 clade sequences (3.7%), Cycloclasticus (2.3%), and Alteromonas (1.8%) (Fig. 2). Among the epsilon-proteobacteria sequences, the genera Sulfurimonas (11.9%), Sulfurovum (4.9%), and Arcobacter (2.1%) were present at relatively high concentrations (Fig. 2a). Additionally, the SAR324 clade (4.4%) of delta-proteobacteria and the SAR202 clade (3.2%) of Chloroflexi were detected in the rising plume sample (Fig. 2).
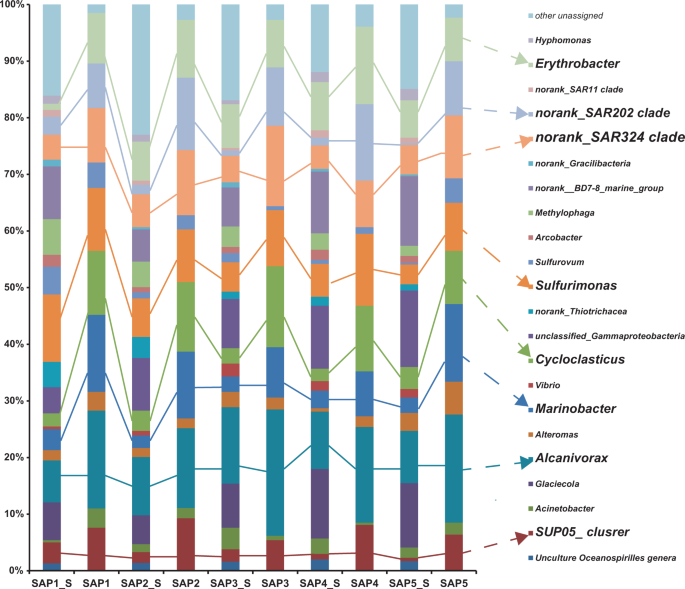
Only genera that represent >3% of the communities in at least one sample are indicated. The “unassigned” categories represent all of the groups comprising <3% of the communities.
In the two neutrally buoyant plume samples (SAP-2_S to 5_S), which were collected far from the vent compared with the samples described above, the sequence reads mainly corresponded to gamma-proteobacteria (34.3–47.2%), epsilon-proteobacteria (5–8.8%), and alpha-proteobacteria (8.1–10.3%) (Fig. 2). The abundance of epsilon-proteobacteria decreased and alpha-proteobacteria appeared. The gamma-proteobacteria were mainly composed of Alcanivorax (9.2–10.3%), Cycloclasticus (2.2–3.9%), Glaciecola (5.1–11.4%), Marinobacter (2.2–3.1%), the SUP05 clade (0.6–2.2%), and Alteromonas (0.6–3.2%), among which the first two are well known for alkane and PAHs degradation. The epsilon-proteobacteria mainly were mainly composed of Sulfurimonas (3.5–6.8%), Sulfurovum (0.3–1.6%), and Arcobacter (0.9–1.8%). Notably, the genus Erythrobacter of alpha-proteobacteria also occurred as a dominant member, accounting for 6.6–8.5% of the total bacterial 16S rRNA gene sequences. In addition, clade SAR324 of delta-proteobacteria and clade SAR202 of Chloroflexi also occurred as the dominant bacteria in situ, comprising 4.1–5.8% and 0.9–1.7% of the total 16S rRNA sequence reads, respectively.
Hydrocarbon biodegradation in the hydrothermal plume
To confirm the bacteria were utilizing hydrocarbons from the hydrothermal plumes, enrichment with a mixture of n-alkanes and PAHs as the carbon and energy sources was conducted while mimicking the deep-sea conditions of high static pressure and low temperature. Quantification showed that both alkanes and PAHs could be degraded significantly by the five plume-derived enrichment consortia (SAP-1 to SAP-5) under 20 MPa and at 10 °C. Specifically, nearly all n-alkanes were removed after 60 days, with the degradation percentages of 91.7–96.5% (Table S3), while 74.6–84.1% of the total PAHs were removed (Table S3).
Further, bacteria capable of hydrocarbon degradation were retrieved by SIP-sequencing. Four genera of bacteria were enriched with alkanes and the PAHs mixture as follows: Alcanivorax (14.1–22.3%), Marinobacter (7.9–13.1%), Cycloclasticus (9.4–14.3%), and Erythrobacter (7.7–13.7%) (Fig. 2). Unexpectedly, the previously recognized chemoautotrophic bacteria were also retained in all of the SIP communities, including the genus Sulfurimonas and the SUP05 and SAR324 clades.
Bacterial diversity in hydrothermal chimneys
The detailed bacterial diversity of black smoker chimney samples collected from three active hydrothermal fields in the SWIR, SMAR, and EPR are shown in Fig. 3. Despite the great geographic distance, the bacterial compositions at the three sites were similar, being mainly composed of Sulfurovum, Sulfurimonas, Thiomicrospira, Nitrospira, Desulfurobacterium, Thermodesulfatator, Desulfobulbus, Pseudoalteromonas, Marinicella, Gallionella, Marinobacter, Halomonas, and Alcanivorax, although they did vary to some extent. Additionally, the SAR202 clade was prevalent the indigenous consortia of all three sulfide chimneys.
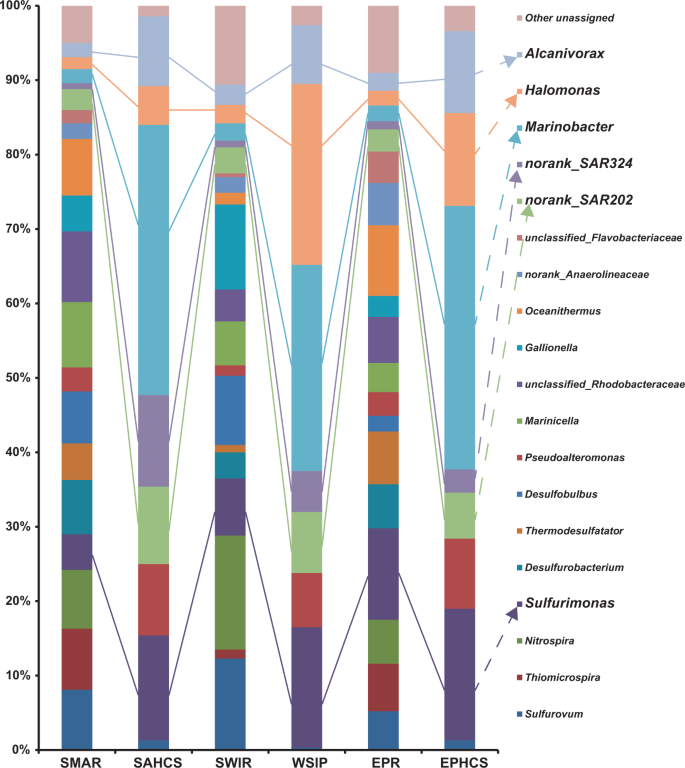
Only genera that represented >3% of the communities in at least one sample are indicated. The “unassigned” categories represented all of the groups comprising <3% of the communities.
Hydrocarbon-degrading bacteria from the hydrothermal chimneys
To identify the hydrocarbon-degrading microbes inhabiting the hydrothermal chimneys, enrichment with a hydrocarbon mixture was conducted under hydrostatic pressures (20–35 MPa) and 10 °C according to the water depths and in situ temperatures, after which the hydrocarbon biodegradation capability of the hydrothermal chimney-derived consortia was tested. Within 20 days, 55–66% of the total n-alkanes and 20–35% of the total PAHs had been degraded. At day 40, 77–89% of the total n-alkanes and 44–52% of the total PAHs had been degraded, while at day 60, 92–97% of the total n-alkanes and 76–78% of the total PAHs had been degraded (Table S4). To define what types of bacteria in hydrothermal chimneys were hydrocarbon degraders, SIP-Seq data analysis was conducted after enrichment with 13C-labeled hydrocarbons for 60 days under 30 MPa at 10 °C. The results revealed that the SIP community mainly contained the following genera: Marinobacter (27.7–36.3%), Sulfurimonas (14.1–17.7%), Halomonas (5.2–24.3%), and Pseudoalteromonas (7.3–9.6%), as well as bacteria of clade SAR202 (6.2–10.4%) and clade SAR324 (3.1–12.3%). Among these, Marinobacter had the highest abundance, followed by Sulfurimonas and Halomonas. Congruent with the in situ diversity, despite the large geographic distance, quite similar degrading bacterial communities were obtained from the three global ocean hydrothermal chimney samples. Detailed compositions of the above hydrocarbon-degrading consortia are shown in Fig. 3.
Bacterial diversity in hydrothermal sediments
Bacterial compositions of the five hydrothermal sediment samples collected from the SWIR, SMAR, and EPR sites are shown in Fig. 4. The following bacteria were characterized as the dominant members in the five sediments based on their 16S rRNA gene abundance: Nitrospira (6.3–9.1%), Erythrobacter (1.8–4.9%), Burkholderia (2.3–9.3%), Alcanivorax (1.4–3.9%), Marinobacter (1.5–3.4%), Rhodococcus (0.5–3.5%), Halomonas (1.3–3.2%), Pseudomonas (0.7–3.9%), Pusillimonas (0.5–3.5%), Acinetobacter (0.6–1.8%), and the SAR202 clade (0.7–2.7%) (Fig. 4). In addition, the bacterial no-rank OM1 clade, S085 clade, JG30-KF-CM66 clade, Acidobacteria, Anaerolineaceae, Hyphomicrobiaceae, Rhodospirillaceae, Aminicenantes, Gemmatimonadetes, Gemmatimonadaceae, and unclassified gamma-proteobacteria frequently occurred in different sediment samples (Fig. 4).
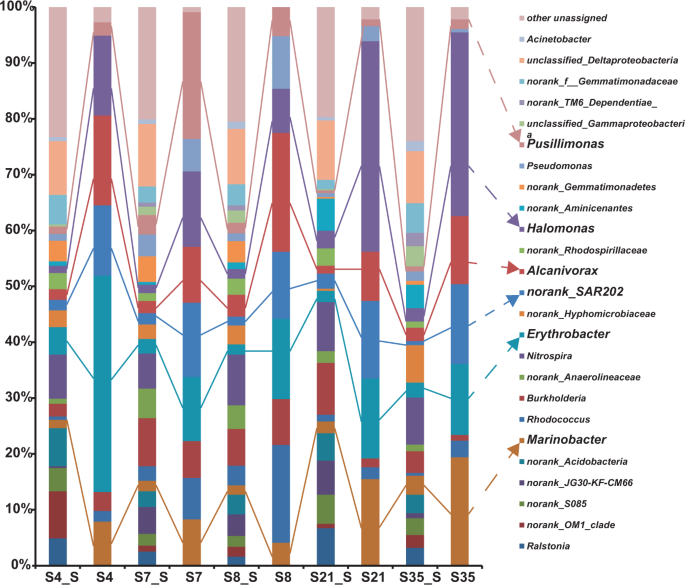
Only genera that represent >3% of the communities in at least one sample are indicated. The “unassigned” categories represent all of the groups comprising <3% of the communities.
Bacterial PAHs biodegradation in hydrothermal sediments
All five hydrothermal sediment consortia enriched with PAHs showed obvious degradation activity under 35 MPa and at 10 °C (Table S5). In addition, all five types of added PAHs of 2–5 rings were significantly degraded. At day 90, naphthalene was totally degraded, while 82–89% of fluoranthene, 87–91% of phenanthrene, 80–94% of pyrene, and 38–50% of benzo[a]pyrene was degraded (Table S5).
To identify the key PAH degraders among the associated bacteria, the above SIP samples were enriched with the PAH mixture for 90 days under 35 MPa and 10 °C, then further processed by SIP-Seq. The results revealed nine bacterial genera were retained as 13C-labeled bacterial members in all degradation communities of the five sediment samples; namely, Erythrobacter, Halomonas, the SAR202 clade, Alcanivorax, Marinobacter, Burkholderia, Pseudomonas, Pusillimonas, and Rhodococcus (Fig. 4). However, the abundance of these organisms varied among samples, with the first four being the most abundant members. Nevertheless, these labeled bacteria were all suggested to be key PAH degraders, such as Pusillimonas and Rhodococcus in the two SWIR consortia S7 and S8 and Marinobacter in consortium SMAR (S21) and EPR (S35).
Isolation of chemoautotrophs from enrichment cultures
The once recognized chemoautotrophic bacteria, including the SAR324 clade, SUP05 clade, and Sulfurimonas, occurred as dominant members of the above hydrocarbon-degrading consortia of vent plumes and sulfides, and were confirmed by SIP-Seq. To better characterize their roles, further enrichment and pure culture isolation and further testing were conducted to determine their potential for hydrocarbon degradation. Thirty-seven out of 1536 culture wells from the above hydrothermal plume and chimney hydrocarbon enrichments were positive for growth, with sulfur oxidation occurring after 21 days. Moreover, four of the cultures had identical 16S rRNA gene sequences and were identified as members of Sulfurimonas, named strain Sulfurimonas sp. hwp1, hwp2, hwp3, and hwp4 (Fig. 5). Two cultures were identified as a delta-proteobacteria related to the SAR324 group, and named SAR324 strain hwp5 and strain hwp6 (Fig. 6). In addition, one culture was identified as a member of the SUP05 clade, named SUP05 strain hwp7 (Fig. 7).
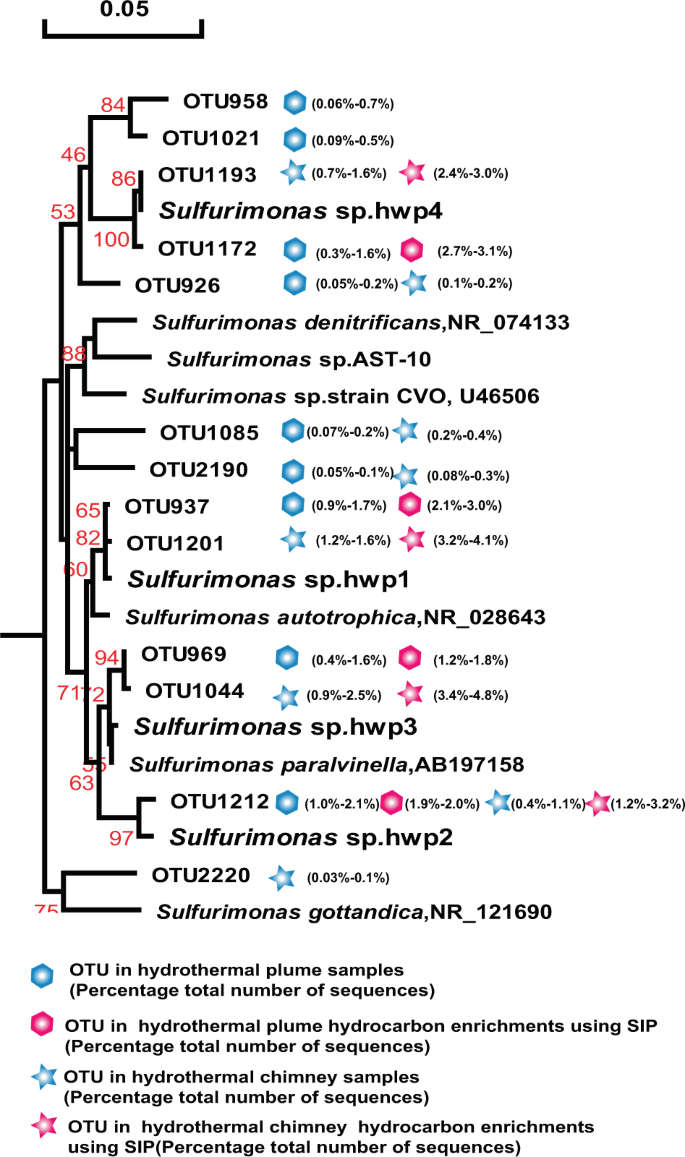
Phylogenetic tree showing the diversity of 16S rRNA gene sequences from OTUs and isolates of Sulfurimonas identified in this study. The tree was constructed using neighbor-joining methods and the Kimura 2-parameter model, as implemented in the MEGA 5.0 software package. The tree is based on partial 16S rRNA gene sequences from this study and their closest type strains. Only bootstrap values ≥50% (based on 1000 bootstrap replicates) are shown at the nodes. The scale bar represents 0.05 nucleotide changes per site. Sulfurimonas OTU distribution among the different hydrocarbon-enrichment consortia and indigenous consortia. Only OTUs representing >1% of the communities in at least one sample are included in the visualization. OTU representative sequences are shown in the Supplementary Information.
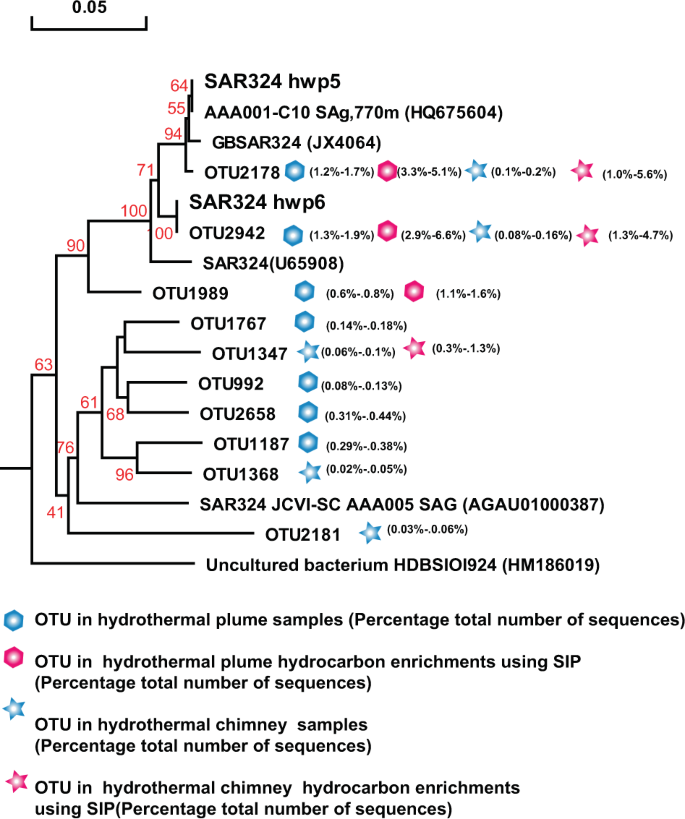
Phylogenetic tree showing the diversity of 16S rRNA gene sequences from OTUs and isolates of SAR324 identified in this study. The tree was constructed using neighbor-joining methods and the Kimura 2-parameter model, as implemented in the MEGA 5.0 software package. The tree is based on partial 16S rRNA gene sequences from this study and their closest type strains. Only bootstrap values ≥50% (based on 1000 bootstrap replicates) are shown at the nodes. The scale bar represents 0.05 nucleotide changes per site. SAR324 OTU distribution among the different hydrocarbon-enrichment consortia and indigenous consortia. Only OTUs representing >1% of the communities in at least one sample are included in the visualization. OTU representative sequences are shown in the Supplementary Information.
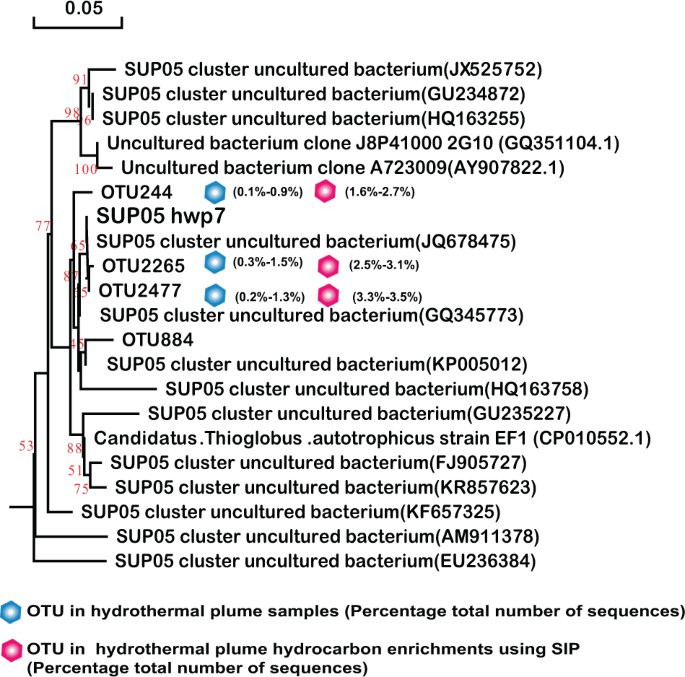
Phylogenetic tree showing the diversity of 16S rRNA gene sequences from OTUs and isolates of SUP05 identified in this study. The tree was constructed using neighbor-joining methods and the Kimura 2-parameter model, as implemented in the MEGA 5.0 software package. The tree is based on partial 16S rRNA gene sequences from this study and their closest type strains. Only bootstrap values ≥50% (based on 1000 bootstrap replicates) are shown at the nodes. The scale bar represents 0.05 nucleotide changes per site. SUP05 OTU distribution among the different hydrocarbon-enrichment consortia and indigenous consortia. Only OTUs representing >1% of the communities in at least one sample are included in the visualization. OTU representative sequences are shown in the Supplementary Information.
Hydrocarbon degradation by chemolithoautotrophs
The hydrocarbon degradation potential of pure cultures of Sulfurimonas spp., and the SAR324 and SUP05 clades were evaluated further under high HPs and low temperatures using hydrocarbon as the sole carbon and energy sources (adequate O2, remove CO2 and thiosulfate). All four isolates of Sulfurimonas and two isolates of SAR324 that were tested exhibited vigorous growth in the short chain length alkane assays using 13C-labeled n-hexane and n-octane under high HPs and low temperatures (Table 1 and Fig. S2). In addition, they also exhibited growth in the medium chain length alkanes of 13C-labeled n-decane and n-dodecane under high pressure and low temperature, but this was comparatively weak (Table 1 and Fig. S2). Only the isolates of SAR324 (strain hwp5 and hwp6) exhibited weak growth in the 13C-labeled n-hexadecane (Table 1 and Fig. S2). All four Sulfurimonas isolates exhibited weak growth under high HPs and low temperatures in the PAH assays using 13C-labeled naphthalene and phenanthrene, respectively (Table 1 and Fig. S2). None of the isolates grew with n-tetracosane, pyrene, or benzo(a)pyrene (Table 1). None of all tested hydrocarbons could be utilized by isolate hwp7 of SUP05 (Table 1).
All four Sulfurimonas isolates exhibited hydrocarbon degradation and degrade n-alkanes of C6–C16 as well as naphthalene and phenanthrene (Table 1). These phylotypes were closely related to the predominant members (>99.7% homology with 450 bps 16S rRNA) in plumes and sulfides in situ as well as those in the hydrocarbon-degrading consortia. Based on the OTU sequences and our isolates, a phylogenetic tree was constructed with references of the type strains (Fig. 5). The results showed that the four isolates were affiliated with four species, among which strain hwp4 isolated from the chimney of the new hydrothermal field Deyin in the southern MAR represented a novel species forming a separate cluster with the homologue OTU1193 from the same chimney sample and OTU1172 from the plume also located at Deyin (pink in Fig. 5), in addition to other members identified in situ (labeled blue). Moreover, strain hwp2 represented a novel species with 96.6% homology to the full-length 16S of Sulfurimonas paralvinella, whereas the strain hwp3 and hwp4 belonged to Sulfurimonas autotrophica and S. paralvinella, respectively. Despite the significant divergence in phylogeny, all four isolates possessed the same characteristics with respect to hydrocarbon utilization.
Similarly, SAR324 isolates hwp5 and hwp6 also exhibited positive alkane degradation (Table 1). Both were isolated from the Deyin field plume and shared 97.85% homology of the 16S rRNA gene, representing two potential novel species. A phylogenetic tree was constructed based on the 16S rRNA gene sequences of this study and the other four sequences retrieved from GenBank (Fig. 6). The phylogenetic result showed that this group in our study samples was quite diverse. The two isolates correspond to the predominant members represented by OTU2178 in the MAR plume and OTU2943 in the MAR sulfides and hydrocarbon-degrading consortia, respectively (Fig. 6). Among the tested n-alkanes from C6 to C32, both isolates could grow with n-hexane, octane, decane, dodecane, and hexadecane (Table 1), while they failed to grow with all tested odd alkanes of C11, C15, and C17, or with long-chain n-alkanes above C20 (data not shown). These isolates could not grow with any tested PAHs either (Table 1). Interestingly, SUP05 isolate hwp7 was closely related to the predominant members in plumes in situ, as well as the hydrocarbon-enrichment consortia, represented by OTU2265 and OTU2477 (Fig. 7); however, this organism was negative for hydrocarbon degradation (Table 1). Our isolate hwp7 together with other OTUs formed an independent cluster in the tree, neighboring with the cluster represented by the previously reported isolate Candidatus Thioglobus autotrophicus strain EF1 (Fig. 7). The two isolates showed 97.5% identity in the full-length 16S rRNA gene sequence, indicating they represent two potential novel species of the genus Thioglobus.
Hydrocarbon degradation by heterotrophic isolates under high pressures
A total of 126 heterotrophic strains were isolated from the above hydrocarbon-enrichment consortia, and most could grow with hexadecane or PAH as the sole carbon and energy source. These organisms were affiliated with 16 genera, including Alcanivorax, Acinetobacter, Alteromonas, Bacillus, Citreicella, Dietzia, Erythrobacter, Halomonas, Idiomarina, Marinobacter, Microbacterium, Novosphingobium, Sphingobium, Pseudomonas, Pusillimonas, and Spongibacter (Table S6).
Thirteen of the isolates could grow with 13C-labeled n-hexadecane as the sole energy source under 35 MPa at 10 °C as determined by cell density OD600 (Fig. S3B), and the degradation was confirmed by quantification using the 13C isotope (Fig. S3A). Based on their 16S rRNA sequences, these isolates were identified as Alcanivorax dieselolei strain S19-9, Alcanivorax sp. strain YLF38, Alcanivorax venustensis strain RY-9, Bacillus safensis strain S21-L1, Halomonas titanicae strain RY-7, Marinobacter bryozoorum strain TG8-3, Marinobacter hydrocarbonoclasticus strain S19-1, Marinobacter segnicrescens strain S19-13, Marinobacter vinifirmus strain S19-10, Oceanicola marinus strain 22F16, Oceanicola nanhaiensis strain 1F26, Rhodococcus yunnanensis strain YLF8, and Pusillimonas sp. strain S7-N8 (Fig. S3A, B).
In the PAH assays, eight isolates exhibited noticeable growth under high HP and low temperature within 60 days based on 13C6-labeled phenanthrene analysis (Fig. S3C), while significant degradation occurred within 120 days under the same conditions (Fig. S3D). These isolates were identified and named as Erythrobacter sp. strain S35-N8, Erythrobacter sp. strain S21-N3, Pusillimonas sp. strain S7-N8, E. flavus Y strain LF25, Marinobacter algicola strain YLF36, M. hydrocarbonoclasticus strain S19-1, B. safensis strain S21-L1, and B. safensis strain S8-L9.
Interestingly, Erythrobacter sp. S21-N3 and Pusillimonas sp. S7-N8 exhibited vigorous growth using various PAHs including naphthalene–13C6, phenanthrene–13C6, pyrene–13C6, fluorene–13C6, and benzo[α]pyrene–13C6 (Figs. S3E, F, and S4).
Source: Ecology - nature.com


