Individual and combined effects of pyraclostrobin and fipronil on head proteome of nurse honeybees
The changes in the head proteome of nurse honeybees exposed to environmentally relevant doses of the fungicide pyraclostrobin, insecticide fipronil (850 and 2.5 ppb, respectively), and combinations of the two (pyraclostrobin + fipronil) were examined using the 2D-PAGE technique and compared with the proteome of bees not exposed to pesticides (control). Bees were exposed to pesticides in colonies where they received pollen patties contaminated for 6 d ad libitum (see Methods for details).
Image analysis of four colloidal Coomassie-stained 2D-PAGE gels for each treatment were analysed using Image Master 2-DE Platinum 7.0 software. Results showed 227 protein spots in the control group and 215, 221, and 211 spots in bees exposed to pyraclostrobin, fipronil, and pyraclostrobin + fipronil, respectively. A high scatter plot correlation coefficient was obtained between the four gels obtained in each treatment (98–100%). Protein spots were displayed within the isoelectric point (pI) range of 4.07–9.93 and mass range of 9.66–136.1 kDa. Gels from bees exposed to pesticides were individually subject to pairwise comparisons to the control group to deduce the fold expression level of proteins. For this, the relative volume parameter (%Vol) was used, which has been reported to be an efficient measure because it takes into account variation due to protein loading and staining by considering the total volume over all the spots in the gel29,30,31.
When we compared protein expression between bees exposed to pyraclostrobin and the control group, 55 of the analysed spots were accepted as significantly differentially expressed between the two experimental conditions (10 were upregulated, 33 were downregulated, and 12 were uniquely expressed in the control group). In the analysis between bees exposed to fipronil and the control group, 56 of the analysed spots were differentially expressed (6 were upregulated, 44 were downregulated, and 6 were uniquely expressed in the control group). When we compare bees exposed to pyraclostrobin + fipronil and control group, 74 of the analysed spots were differentially expressed (15 were upregulated, 43 were downregulated, and 16 were uniquely expressed in the control group) (Fig. 1). Interestingly, in bees exposed to pyraclostrobin + fipronil, we observed higher fold-changes in the expression of spots that were up- and downregulated; additionally, the number of spots not present in bees exposed to this combination of pesticides was higher (Supplementary Tables S1 and S2).
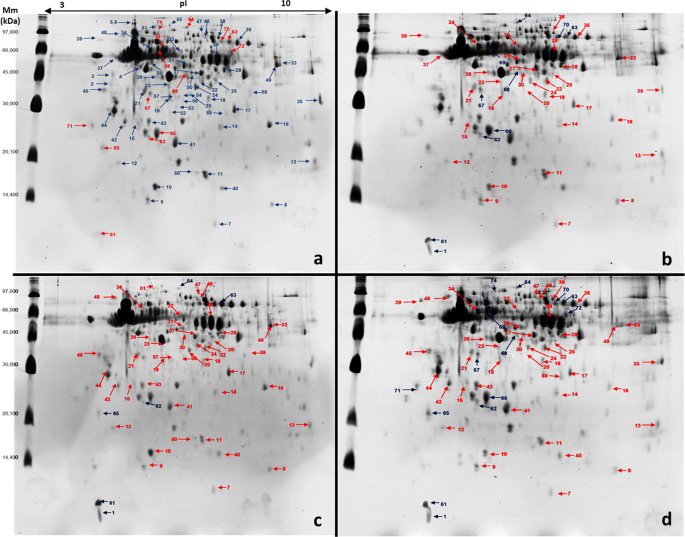
Representative 2D-PAGE gels from nurse honeybee heads (Apis mellifera). Bees exposed to field-relevant doses of pyraclostrobin and fipronil individually and combined. First dimension: 375 μg whole protein extracts from a pool of 30 honeybee heads on immobilised pH 3–10 nonlinear gradient strips (proteins were extracted in duplicate). Second dimension: 12.5% SDS-PAGE gels (two gels for each protein extract). Numbers represent ID of spots accepted as significantly differentially expressed between control and pesticide exposure treatments (see Supplementary Tables S1 and S2 for more details). Red spots represent decreased expression, and blue spots represent increased expression. (a) Control, (b) pyraclostrobin, (c) fipronil, and (d) pyraclostrobin + fipronil treatments.
All protein spots with significant changes in expression in bees exposed to pesticides and those found only in the control group were manually excised in gel digested with trypsin and analysed using ESI-MS/MS. In these analyses, 93 proteins were identified in spots downregulated in bees exposed to pesticides and 19 proteins in upregulated spots. Twenty-one proteins were identified in spots uniquely expressed in the control group, and 15 of these same proteins were also found in spots downregulated in bees exposed to pesticides. Only in four spots were the proteins unidentified due to their values being too low to produce a spectrum, or because the C.I.% of the database search was not higher than 95%, which was needed to yield unambiguous results.
Some proteins were identified in different protein spots possibly due to post-translational modifications that altered their molecular mass (Mm) and pI32, and more than one protein was identified in a single spot, as also observed in previous studies31,33,34. The identification of more than one protein in a single spot may be due to post-translational modifications35 or proteins belonging to multigenic families or isoforms, which generally have similar Mm and pI36.
Protein sequences were analysed using the Blast2GO tool to annotate with Gene Ontology (GO) terms and subsequent identification of the metabolic pathways in the Kyoto Encyclopedia of Genes and Genomes (KEGG), which allowed the separation of functional groups (Table 1).
In general, the analysis of protein sequences identified in spots downregulated in bees exposed to pesticides in Blast2GO using GO resulted in scores mainly associated with the extracellular region, cytoplasm part, and protein complex and for the molecular functions of protein binding, transferase activity, metal ions binding, and ATP binding and for the biological processes of oxidation reduction, transport, cellular protein metabolism, and glycolysis (Fig. 2). Analysis of protein sequences identified in spots upregulated in bees exposed to pesticides resulted in scores mainly associated with the extracellular region, the molecular function of ATP binding, and various biological processes (Supplementary Fig. S1). These analyses demonstrated that exposure of nurse bees to pyraclostrobin and fipronil individually and in combination at field-relevant doses promoted changes in metabolism that may reduce the expression of proteins and increase their susceptibility to these molecules and other stressors, such as diseases, parasites, and other pesticides.
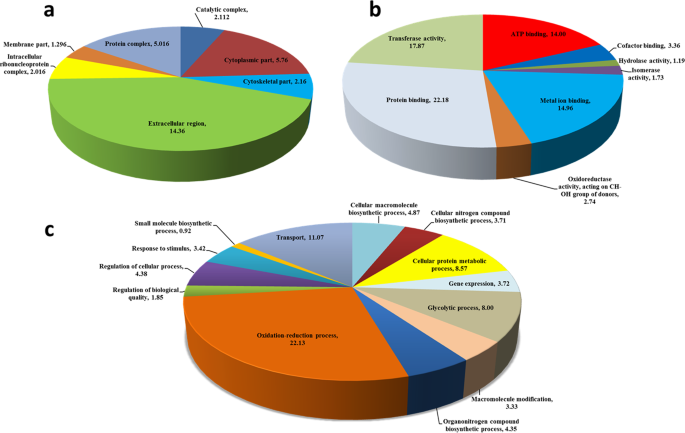
Classification of the protein sequences showing decreased expression (p < 0.05) in nurse bees (Apis mellifera) exposed to field-relevant doses of the fungicide pyraclostrobin and insecticide fipronil. (a) Cellular component, (b) molecular function, and (c) biological process.
Expression of major royal jelly proteins in nurse honeybees exposed to pesticides
The major RJ proteins (MRJPs) MRJP1, MRJP2, MRJP4, and MRJP5 were identified in spots that were downregulated in nurse bees exposed to pesticides or that were uniquely expressed in the control group. MRJP expression further decreased under treatment with pyraclostrobin + fipronil (Supplementary Table S1). Previous studies have reported that exposure of nurse honeybees to diets contaminated with pesticides caused morphological changes in hypopharyngeal glands9,37,38, and in both hypopharyngeal and mandibular glands15 that are responsible for the synthesis of RJ.
These studies suggested that morphologic alterations in these glands could quantitatively and qualitatively decrease the secretion of proteins in RJ. To our knowledge, our study is the first to demonstrate potential alterations in RJ protein produced by nurse honeybees exposed to pesticides. Nurse bees with impaired brood-food glands can potentially forage precociously, and this can reduce their life span39.
RJ proteins are key factors in colony nutrition, and antimicrobial defence22,40. Larvae destined to be queens receive only RJ during their development and adult life, whereas pollen and honey are added to the diet of worker-destined larvae22. The decrease in MRJP1 expression observed in our study is important because this protein has antimicrobial properties that are essential for larval health and decreasing susceptibility to pathogens40,41. Previous studies have reported impaired colony growth and queen production16, higher queen replacement rates17, decreased immunity42, and altered physiological development of queens43 that are reared in colonies exposed to pesticides. Our results suggest that these factors may be associated with changes in the quality of the RJ produced by nurse honeybees that consume pollen contaminated with pesticides. Future studies to evaluate the quality and quantity of RJ produced directly in colonies exposed to pesticides are necessary to confirm the effects observed in our study and to better understand the effects of pesticide exposure on larval nutrition, caste differentiation, and colony maintenance.
General changes in metabolism of nurse honeybees exposed to pesticides
We identified important proteins involved in carbohydrate metabolism and energy synthesis (11 proteins), antioxidant system (18 proteins), and biosynthesis (seven proteins) in spots downregulated in nurse honeybees exposed to pesticides (Supplementary Table S1). These alterations characterise important changes in nurse honeybee metabolism. Once under normal conditions, nurse bees exhibited increased expression of proteins involved in these functions to perform their tasks in the colony32. Other important proteins that were downregulated were associated with amino acid metabolism, transcription/translation, protein binding/folding, olfactory system, learning and memory, and other proteins with other or unknown functions (Supplementary Table S1).
Downregulation of enzymes involved in carbohydrate and energy metabolism: effect on ATP production
Reduction and/or absence of expression of the enzymes fructose-bisphosphate aldolase [Enzyme Commission (EC) number 4.1.2.13], triosephosphate isomerase (EC 5.3.1.1), phosphoglycerate mutase (EC 5.4.2.12), malate dehydrogenase (EC 1.1.1.37), aldose 1-epimerase (EC 5.1.3.3), glucose-6-phosphate isomerase (EC 5.3.1.9), and phosphomannomutase (EC 5.4.2.8) which participate in the processes of glycolysis and gluconeogenesis, and also the enzymes inorganic pyrophosphatase (EC 3.6.1.1), arginine kinase (EC 2.7.3.3), transaldolase (EC 2.2.1.2), and phosphoglycolate phosphatase-like (EC 3.1.3.18), which act in carbohydrate metabolism and feed into glycolysis pathway, characterise important changes in bees exposed to pesticides.
Decreased expression of these enzymes can impair ATP synthesis and lead to lower levels of the energy necessary to maintain protein biosynthesis, which may compromise RJ synthesis in nurse bees. Energy depletion may also impair detoxification processes that require high energy expenditure44,45,46. We also observed a decrease in the expression of arginine kinase, which is responsible for transporting ATP and is required for biochemical processes of the visual system, such as the regeneration of pigments in the retina29. According to Roat et al.29, who also observed decreased expression of this enzyme in bees exposed to fipronil, its reduction may impair vision in bees.
Our results corroborate those of previous studies that demonstrated impairment of energy metabolism in bees exposed to pesticides47,48. Suppression of ATP production by pyraclostrobin occurs by inhibiting the ubiquinol-oxidation centre of the mitochondria1 bc1 complex that blocks the electron transfer between cytochrome b and cytochrome c1 and disrupts ATP production49. Fipronil may impair ATP synthesis in bees due to the inhibition of the electron transport chain50. Mao et al.48 reported that bees exposed to fungicides did not metabolise some of the phytochemicals naturally present in pollen, and these molecules can compromise ATP synthesis. The expression of enzymes that act in glycolysis and gluconeogenesis is regulated, which limits their functions and activity aimed at meeting their metabolic rate51. Therefore, the inhibitory action of fipronil and pyraclostrobin on the electron transport chain and a possible influence of phytochemicals may have contributed to the decreased expression of enzymes involved in ATP synthesis. An additive effect, characterised by a further decrease in the expression of some enzymes involved in energy synthesis was observed in bees exposed to pyraclostrobin + fipronil. This result may be derived from the interaction between pesticides and nonmetabolised phytochemicals in pollen.
Downregulation of antioxidant enzymes and increase in susceptibility to oxidative stress, pesticides, and diseases
We identified the following important enzymes: superoxide dismutase [Cu-Zn], peroxiredoxin 1, pyridoxine pyridoxamine 5-phosphate oxidase, 15-hydroxyprostaglandin dehydrogenase [NAD(+)]-like, lambda-crystallin homolog, trans-1,2-dihydrobenzene-1,2-diol dehydrogenase-like, glycerol-3-phosphate dehydrogenase [NAD(+)], malate dehydrogenase, aldose reductase-like, glycerol-3-phosphate dehydrogenase [NAD(+)], and probable medium-chain specific acyl-mitochondrial in downregulated spots in nurse bees exposed to pyraclostrobin + fipronil. Additionally, the enzymes glutathione S-transferase S1 and 3-hydroxyacyl- dehydrogenase type-2 were identified in downregulated spots in bees exposed to pyraclostrobin and pyraclostrobin + fipronil, and the enzymes 15-hydroxyprostaglandin dehydrogenase [NAD(+)]-like, Rab s geranylgeranyltransferase component A1, glutathione peroxidase, thioredoxin domain-containing 9, farnesol dehydrogenase-like, and prophenoloxidase (PO) were identified in downregulated spots in nurse bees exposed to fipronil and pyraclostrobin + fipronil.
All protein synthesis processes in eukaryotes demand high levels of ATP52, which consequently increases the production of reactive oxygen species (ROS)32,53. During ROS generation in cells, the expression of antioxidant enzymes increases to maintain homeostasis46,54. Our results demonstrated the downregulated antioxidant proteins in nurse bees exposed to pesticides that can increase their susceptibility to oxidative stress and were characterised by increased ROS generation and/or reduced physiological activity of antioxidant enzymes55. Some pesticides can act as pro-oxidants by impairing the functionality of antioxidants56 and to bees that presents a smaller number of genes that encode antioxidant proteins compared with the genomes of other insects53. This may represent an additional barrier to their defence against ROS53,57.
Higher susceptibility of nurse bees to oxidative stress may lead to damaged macromolecules58, DNA and RNA oxidation, lipid membrane peroxidation59, and impaired development of glands and decreased longevity58. Our previous study reported a reduction in the development of mandibular and hypopharyngeal glands in nurse honeybees exposed to pyraclostrobin and fipronil individually and in combination15. Other previous studies reported different responses of antioxidant system of honeybees exposed to pesticides or natural compounds, and these responses can change based on the age of the bee, time of exposure, dosage, and pesticide group46,47,54,60,61.
The enzyme superoxide dismutase [Cu-Zn] represents the first line of defence against ROS generated in mitochondria. This enzyme acts in the cytoplasm and converts superoxide radicals to oxygen and hydrogen peroxide, that are decomposed by catalases and peroxiredoxins, as peroxiredoxin 153. An increase in superoxide dismutase isoforms62 and glutationes (GSTs) enzymes is associated with increased insect resistance to pesticides63,64. We found that these antioxidant enzymes were downregulated in bees exposed to fipronil, pyraclostrobin, and pyraclostrobin + fipronil that can increase the sensibility of these insect to these pesticides.
We also observed the downregulation of PO in bees exposed to fipronil without a synergic or addictive effect on their expression in bees exposed to combined pesticides, which suggests that fipronil is the main pesticide responsible for this effect. PO is an enzyme associated with immune response in bees and is involved in melanogenesis in invertebrates65,66, a key process in defence against bacteria, fungi, and viruses65. Thus, PO downregulation in bees exposed to fipronil can decrease the ability of bees to defend against microorganisms and increase their susceptibility to disease. A study by Zhu et al.66 reported that PO activity was reduced in bees exposed to pesticide mixtures.
Downregulation of enzymes involved in biosynthesis and amino acid metabolism: implications for prostaglandin biosynthesis and detoxification
In nurse bees exposed to pyraclostrobin and fipronil we observed a decrease in the expression of the enzymes prostaglandin E synthase 3 (EC 5.3.99.3); pyridoxamine 5′-phosphate oxidase (EC 1.4.3.5); glutamine synthetase 2 cytoplasmic isoform X1 e glutamine synthetase 2 cytoplasmic (EC 6.3.1.2), involved in glutamine and arginine biosynthesis; 6-pyruvoyl tetrahydrobiopterin synthase (EC 4.2.3.12) involved in folate biosynthesis; alanine aminotransferase 1 (EC 2.6.1.2), involved in arginine biosynthesis; and hydroxyacylglutathione mitochondrial isoform X1 (EC 3.1.2.6) involved in glutathione biosynthesis.
Decreased expression of prostaglandin E synthase 3 can affect prostaglandin biosynthesis, which acts on reproduction, modulation of fluid secretion in glands, and in insect immune response67. The enzyme pyridoxamine 5′-phosphate oxidase is involved in the de novo synthesis of pyridoxine and pyridoxal phosphate, which are found in RJ and are essential to larval development of bees19.
The glutamine that is converted to glutamate is essential for glutathione biosynthesis and allows the functioning of enzymes in the class glutathiones (GSTs), the synthesis of polyamines that interact with DNA/RNA, and protein biosynthesis54. The downregulation of proteins that are involved in glutamine and glutathione biosynthesis reflects the decreased expression of GST enzymes observed in bees exposed to fipronil, pyraclostrobin, and pyraclostrobin + fipronil, indicating an indirect reduction in their detoxification ability. Additionally, the downregulation of protein-L-isoaspartate O-methyltransferase (EC 2.1.1.77) and s-methyl-5′-thioadenosine phosphorylase (EC 2.4.2.28) involved in cysteine and methionine metabolism, and alanine aminotransferase 1 (EC 2.6.1.2), glutamine synthetase 2 cytoplasmic isoform X1, and glutamine synthetase 2 cytoplasmic (EC 6.3.1.2) involved in alanine, aspartate, and glutamate metabolism (except alanine aminotransferase 1 to pyraclostrobin) also support the hypothesis of decreased detoxification ability in bees exposed to fipronil, pyraclostrobin, and pyraclostrobin + fipronil. Amino acid catabolism contributes to energy generation and can generate Krebs cycle intermediates used as precursors for the synthesis of molecules such as glutathione that are involved in detoxification54.
Downregulation of enzymes involved in transcription/translation
The proteins ubiquitin-60S ribosomal L40, eukaryotic translation initiation factor 3 subunit I, elongation factor 1-gamma, and tyrosine–tRNA ligase were identified in downregulated spots in bees exposed to fipronil, pyraclostrobin, and pyraclostrobin + fipronil. The enzyme U3 small nucleolar RNA-associated 4 homolog A was identified in spots that presented downregulation in bees exposed to fipronil and to association of pesticides, and the protein eukaryotic initiation factor 4A-I was identified in spots downregulated in bees exposed to association of pesticides. These proteins have functions involved in RNA translation and/or transport processes68 and their downregulation suggests a reduction of protein synthesis in bees exposed to pesticides.
Downregulation of enzymes involved in protein binding/folding
The proteins hsp70-binding 1, 10 kda heat shock mitochondrial, profilin, t-complex 1 subunit eta, and 60 kda heat shock mitochondrial-like have protein binding functions, such as chaperone activity or in protein folding processes that are essential for maintaining the functionality of proteins, and their expression decreased in all groups exposed to pesticides. These results suggest that proteins with essential functions pertaining to the conformation and adequate functioning of other proteins can be affected by the exposure of bees to pesticides. Proline binds to actin and has important structural functions. The 60 kda heat shock mitochondrial-like 6 protein is involved in protein folding and processing of genetic information and RNA, guaranteeing the turnover of RNA molecules, a process that is critical for gene expression68.
Downregulation of proteins involved in olfaction, learning, and memory
The expression of the protein odorant-binding protein 14 (OBP14) was decreased in all bees exposed to pesticides. The odorant proteins are required for the adequate recognition of chemical stimuli by the insect olfactory system69, and decreased expression can impair the perception of stimuli by nurse bees and affect their functionality in the colony. The proteins 14-3-3 epsilon and 14-3-3 zeta that are involved in learning and memory in insects70,71 were also downregulated in bees exposed to pesticides. These results suggest an impairment of these functions in bees due to exposure to fipronil, pyraclostrobin, and pyraclostrobin + fipronil.
Proteins upregulated in bees exposed to pesticides
Some proteins involved in carbohydrate metabolism and energy synthesis [malic enzyme (EC 1.1.1.40), pyruvate dehydrogenase E1 component subunit mitochondrial (EC 1.2.4.1), and pyruvate kinase (EC 2.7.1.40)], others involved in protein synthesis (eukaryotic translation initiation factor 3 subunit K), and some proteins with other or unknown functions were identified in spots upregulated in bees exposed to pesticides (Supplementary Table S2).
We observed an increase in the expression of heat shock 70 kda cognate 5, heat shock 70 kda 4 isoform X1, and heat shock 70 kda cognate 3, which are in the heat shock protein (HSP) family. These proteins are cellular markers of stress response, and an increase in these proteins in Drosophila melanogaster and bees exposed to pesticides has been previously reported37,72. HSPs can prevent cell apoptosis73,74 and protein denaturation and can promote the degradation of abnormal proteins73; however, the effects of their upregulation remains poorly understood. In addition, two isoforms of the protein lethal(2)essential for life–like, which are also in the HSP family and function as chaperones exhibited increased expression in bees exposed to pesticides. A study performed by Roat et al.29 reported increased expression of lethal(2)essential for life–like in bees exposed to fipronil. We suggest that increased expression of HSP proteins and chaperones can be involved in the cytotoxic effects of fipronil and increase the damage to protein structure caused by oxidative stress in bees exposed to pesticides.
Our results demonstrated that exposure of nurse honeybees to field-relevant doses of the pyraclostrobin, fipronil, and pyraclostrobin + fipronil caused changes in vital metabolic processes, and we characterised new effects associated with exposure of bees to pesticides. These changes interfered with nurse bee functions that are essential for the development and maintenance of their colony, increasing the susceptibility of the colony to collapse. Nurse bees with impaired brood-food glands15 and lower capacity of RJ production can potentially forage precociously, and this can reduce their life time39.
The global analysis of protein profiles in nurse honeybee heads with the 2D-PAGE technique allowed us to screen the main changes that occur when bees are exposed to pesticides. However, our results also highlight the necessity for further detailed research to examine changes in protein expression and to evaluate the effects of pesticides on pollinators to detect chronic effects that may compromise colony maintenance and to fully understand the effects of pesticides on honeybee colony functionality. Owing to the negative effects of exposure to pyraclostrobin and fipronil observed in our study, it seems evident that pesticide use for the maintenance of native and managed pollinators should be reduced.
Source: Ecology - nature.com


