Svanbäck, R. & Bolnick, D. I. Intraspecific competition drives increased resource use diversity within a natural population. Proceedings of the Royal Society B: Biological Sciences 274(1611), 839–844 (2006).
Hutchinson, G. E. Concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology 22, 415–427 (1957).
Schoener, T. W. Resource partitioning in ecological communities. Science 185, 27–38 (1974).
Hardin, G. The competitive exclusion principle. Science 131(3409), 1292–1297 (1960).
Staniewicz, A., Behler, N., Dharmasyah, S. & Jones, G. Niche partitioning between juvenile sympatric crocodilians in Mesangat Lake, East Kalimantan, Indonesia. Raffles Bulletin of Zoology 66, 528–537 (2018).
MacArthur, R. & Wilson, E. The Theory of Island Biogeography (Princeton University Press, 1967).
Root, R. The niche exploitation pattern of the blue-gray gnatcatcher. Ecological Monographs 37, 317–350 (1967).
Ratcliffe, N. et al. The roles of sex, mass and individual specialisation in partitioning foraging-depth niches of a pursuit-diving predator. PloS One 8(10), e79107 (2013).
Tschumy, W. O. Competition between juveniles and adults in age-structured populations. Theoretical Population Biology 21(2), 255–268 (1982).
Johst, K., Berryman, A. & Lima, M. From individual interactions to population dynamics: individual resource partitioning simulation exposes the causes of nonlinear intra‐specific competition. Population Ecology 50(1), 79–90 (2008).
Zhao, T., Villéger, S., Lek, S. & Cucherousset, J. High intraspecific variability in the functional niche of a predator is associated with ontogenetic shift and individual specialization. Ecology and Evolution 4, 4649–4657 (2014).
Thiemann, G. W., Iverson, S. J., Stirling, I. & Obbard, M. E. Individual patterns of prey selection and dietary specialization in an Arctic marine carnivore. Oikos 120(10), 1469–1478 (2011).
Carneiro, A. P., Bonnet-Lebrun, A. S., Manica, A., Staniland, I. J. & Phillips, R. A. Methods for detecting and quantifying individual specialisation in movement and foraging strategies of marine predators. Marine Ecology Progress Series 578, 151–166 (2017).
Bon, R. & Campan, R. Unexplained sexual segregation in polygamous ungulates: a defense of an ontogenetic approach. Behavioural Processes 38(2), 131–154 (1996).
Pellegrini, A. D. Sexual segregation in childhood: A review of evidence for two hypotheses. Animal Behaviour 68(3), 435–443 (2004).
Ruckstuhl, K. E. Foraging behaviour and sexual segregation in bighorn sheep. Animal Behaviour 56(1), 99–106 (1998).
Conradt, L. & Roper, T. J. Activity synchrony and social cohesion: a fission-fusion model. Proceedings of the Royal Society of London. Series B: Biological Sciences 267(1458), 2213–2218 (2000).
Gittleman, J. L. & Valkenburgh, B. V. Sexual dimorphism in the canines and skulls of carnivores: effects of size, phylogency, and behavioural ecology. Journal of Zoology 242(1), 97–117 (1997).
Mayer, M., Shine, R. & Brown, G. Bigger babies are bolder: effects of body size on personality of hatchling snakes. Behaviour 153, 313–323 (2016).
Isaac, J. L. Potential causes and life-history consequences of sexual size dimorphism in mammals. Mammal Review 35, 101–115 (2005).
Clutton-Brock, T. H. & Harvey, P. H. The functional significance of variation in body size among mammals. Special Publication of the American Society of Mammalogists 7, 632–663 (1983).
Prins, H. H. T. & Olff, H. Species richness of African grazer assemblages: towards a functional explanation in Dynamics of Tropical Communities (Eds Newbery, D. M., Prin, H. H. T. & Brown, N. D.) 449–490 (Blackwell, 1998).
Ruckstuhl, K. E., Clutton-Brock, T. & Neuhaus, P. Sexual segregation and the ecology of the two sexes (Cambridge University Press, 2005).
Stokke, S. & du Toit, J. T. Sex and size related differences in the dry season feeding patterns of elephants in Chobe National Park, Botswana. Ecography 23(1), 70–80 (2000).
Jakimchuk, R. D., Ferguson, S. H. & Sopuck, L. G. Differential habitat use and sexual segregation in the Central Arctic caribou herd. Canadian Journal of Zoology 65(3), 534–541 (1987).
Lingle, S. Coyote predation and habitat segregation of white‐tailed deer and mule deer. Ecology 83(7), 2037–2048 (2002).
Staniland, I. J. & Robinson, S. L. Segregation between the sexes: Antarctic fur seals, Arctocephalus gazella, foraging at South Georgia. Animal Behaviour 75(4), 1581–1590 (2008).
Heupel, M. R., Carlson, J. K. & Simpfendorfer, C. A. Shark nursery areas: concepts, definition, characterization and assumptions. Marine Ecology Progress Series 337, 287–297 (2007).
Werner, E. E. & Gilliam, J. F. The ontogenetic niche and species interactions in size-structured populations. Annual Review of Ecology, Evolution, and Systematics 15, 393–425 (1984).
Grubbs, R. D. Ontogenetic shifts in movements and habitat use in Sharks and their Relatives II: Biodiversity, Physiology, and Conservation (Eds Carrier, J. C., Musick, J. A. & Heithaus, M. R.) 319–350 (CRC Press, 2010).
Webb, J. K., Shine, R. & Christian, K. A. Does intraspecific niche partitioning in a native predator influence its response to an invasion by a toxic prey species? Austral Ecology 30(2), 201–209 (2005).
Bolnick, D. I. et al. The ecology of individuals: incidence and implications of individual specialization. The American Naturalist 161(1), 1–28 (2002).
Durell, S. E. L. V. D. Individual feeding specialisation in shorebirds: population consequences and conservation implications. Biological Reviews 75(4), 503–518 (2000).
Estes, J. A., Riedman, M. L., Staedler, M. M., Tinker, M. T. & Lyon, B. E. Individual variation in prey selection by sea otters: patterns, causes and implications. Journal of Animal Ecology 72, 144–155 (2003).
Jeglinski, J., Werner, C., Robinson, P., Costa, D. & Trillmich, F. Age, body mass and environmental variation shape the foraging ontogeny of Galapagos sea lions. Marine Ecology Progress Series 453, 279–296 (2012).
Chilvers, B. L. Whisker stable isotope values indicate long-term foraging strategies for female New Zealand sea lions. Endangered Species Research 38, 55–66 (2019).
Beest, F. M. et al. Increasing density leads to generalization in both coarse‐grained habitat selection and fine‐grained resource selection in a large mammal. Journal of Animal Ecology 83(1), 147–156 (2014).
Dayan, T. & Simberloff, D. Patterns of size separation in carnivore communities in Carnivore Behavior, Ecology, and Evolution, vol 2. (Ed. Gittleman, J. L.) 243–266 (Cornell University Press, 1996).
Van Valkenburgh, B. Major patterns in the history of carnivorous mammals. Annual Review of Earth and Planetary Sciences 27, 463–493 (1999).
Costa, A. et al. Generalisation within specialization: inter-individual diet variation in the only specialized salamander in the world. Scientific Reports 5, 13260 (2015).
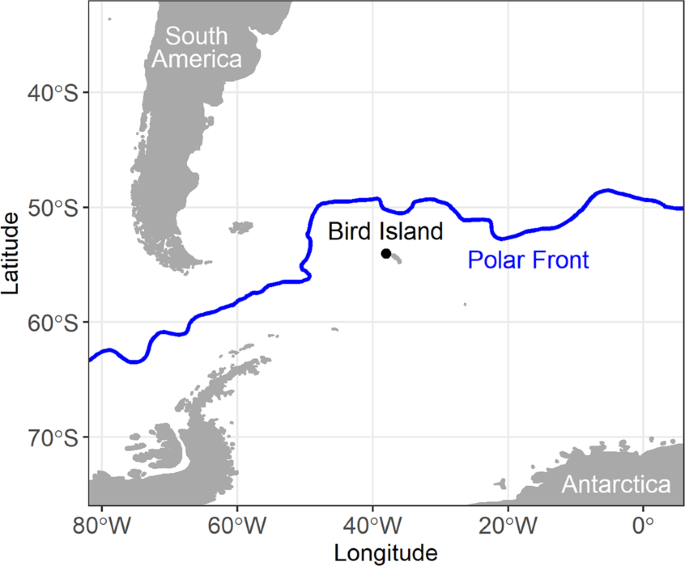
Forcada, J. & Staniland, I. J. Antarctic fur seal Arctocephalus gazella in Encyclopedia of Marine Mammals, 2nd edition (Eds Perrin, W. F., Würsig, B. & Thewissen, J. G. M.) 36–42 (Academic Press, 2009).
Staniland, I. Sexual segregation in seals in Sexual segregation in Vertebrates: Ecology of the Two Sexes (Eds Ruckstuhl, K. E. & Clutton-Brock, T. H.) 53–73 (Cambridge University Press, 2005).
Waluda, C. M., Gregory, S. & Dunn, M. J. Long-term variability in the abundance of Antarctic fur seals Arctocephalus gazella at Signy Island, South Orkneys. Polar Biology 33(3), 305–312 (2010).
Boyd, I. L., McCafferty, D. J., Reid, K., Taylor, R. & Walker, T. R. Dispersal of male and female Antarctic fur seals (Arctocephalus gazella). Canadian Journal of Fisheries and Aquatic Sciences 55(4), 845–852 (1998).
Staniland, I. J., Robinson, S. L., Silk, J. R. D., Warren, N. & Trathan, P. N. Winter distribution and haul-out behaviour of female Antarctic fur seals from South Georgia. Marine Biology 159(2), 291–301 (2012).
Arthur, B. et al. Return customers: Foraging site fidelity and the effect of environmental variability in wide-ranging Antarctic fur seals. PloS One 10(3), e0120888 (2015).
Payne, M. R. Growth in the Antarctic fur seal Arctocephalus gazella. Journal of Zoology 187(1), 1–20 (1979).
Kernaléguen, L. et al. Early-life sexual segregation: ontogeny of isotopic niche differentiation in the Antarctic fur seal. Scientific Reports 6, 33211 (2016).
Lea, M. A. et al. Colony-based foraging segregation by Antarctic fur seals at the Kerguelen Archipelago. Marine Ecology Progress Series 358, 273–287 (2008).
Giménez, J. et al. Intra-and interspecific niche partitioning in striped and common dolphins inhabiting the southwestern Mediterranean Sea. Marine Ecology Progress Series 567, 199–210 (2017).
Newsome, S. D., Martınez del Rio, C., Bearhop, S. & Phillips, D. L. A niche for isotopic ecology. Frontiers in Ecology and the Environment 5, 429–436 (2007).
DeNiro, M. J. & Epstein, S. You are what you eat (plus a few ‰): the carbon isotope cycle in food chains. Geological Society of America 6, 834–835 (1976).
Ben-David, M. & Flaherty, E. A. Stable isotopes in mammalian research: a beginner’s guide. Journal of Mammalogy 93(2), 312–328 (2012).
Minagawa, M. & Wada, E. Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochimica et Cosmochimica Acta 48(5), 1135–1140 (1984).
Fry, B. Food web structure on Georges Bank from stable C, N, and S isotopic compositions. Limnology and Oceanography 33(5), 1182–1190 (1988).
Hobson, K. A., Piatt, J. F. & Pitocchelli, J. Using stable isotopes to determine seabird trophic relationships. Journal of Animal Ecology 63, 786–798 (1994).
Kelly, J. F. Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Canadian Journal of Zoology 78(1), 1–27 (2000).
Goericke, R. & Fry, B. Variations of marine plankton δ13C with latitude, temperature, and dissolved CO2 in the world ocean. Global Biogeochemical Cycles 8(1), 85–90 (1994).
Rau, G. H., Takahashi, T. & Des Marais, D. J. Latitudinal variations in plankton δ13C: implications for CO2 and productivity in past oceans. Nature 341(6242), 516 (1989).
Cherel, Y., Hobson, K. A., Guinet, C. & Vanpe, C. Stable isotopes document seasonal changes in trophic niches and winter foraging individual specialization in diving predators from the Southern Ocean. Journal of Animal Ecology 76(4), 826–836 (2007).
Phillips, R. A., Bearhop, S., Mcgill, R. A. & Dawson, D. A. Stable isotopes reveal individual variation in migration strategies and habitat preferences in a suite of seabirds during the nonbreeding period. Oecologia 160(4), 795–806 (2009).
Cherel, Y., Kernaléguen, L., Richard, P. & Guinet, C. Whisker isotopic signature depicts migration patterns and multi-year intra-and inter-individual foraging strategies in fur seals. Biology Letters 5(6), 830–832 (2009).
Kernaléguen, L. et al. Long-term species, sexual and individual variations in foraging strategies of fur seals revealed by stable isotopes in whiskers. PloS One 7(3), e32916 (2012).
Stowasser, G. et al. Food web dynamics in the Scotia Sea in summer: a stable isotope study. Deep Sea Research Part II: Topical Studies in Oceanography 59, 208–221 (2012).
Moore, J. K., Abbott, M. R. & Richman, J. G. Variability in the location of the Antarctic Polar Front (90–20 W) from satellite sea surface temperature data. Journal of Geophysical Research: Oceans 102(C13), 27825–27833 (1997).
Wakefield, E. D. et al. Long‐term individual foraging site fidelity – why some gannets don’t change their spots. Ecology 96(11), 3058–3074 (2015).
Seyboth, E. et al. Isotopic evidence of the effect of warming on the northern Antarctic Peninsula ecosystem. Deep Sea Research Part II: Topical Studies in Oceanography 149, 218–228 (2018).
Tarroux, A., Lowther, A. D., Lydersen, C. & Kovacs, K. M. Temporal shift in the isotopic niche of female Antarctic fur seals from Bouvetøya. Polar Research 35(1), 31335 (2016).
Hertz, E., Trudel, M., Cox, M. K. & Mazumder, A. Effects of fasting and nutritional restriction on the isotopic ratios of nitrogen and carbon: a meta‐analysis. Ecology and evolution 5(21), 4829–4839 (2015).
Salton, M., Kirkwood, R., Slip, D. & Harcourt, R. Mechanisms for sex-based segregation in foraging behaviour by a polygynous marine carnivore. Marine Ecology Progress Series 624, 213–226 (2019).
Pyke, G. H., Pulliam, H. R. & Charnov, E. L. Optimal foraging: a selective review of theory and tests. The Quarterly Review of Biology 52(2), 137–154 (1977).
Haskell, J. P., Ritchie, M. E. & Olff, H. Fractal geometry predicts varying body size scaling relationships for mammal and bird home ranges. Nature 418, 527–530 (2002).
Boyd, I. L. Estimating food consumption of marine predators: Antarctic fur seals and macaroni penguins. Journal of Applied Ecology 39(1), 103–119 (2002).
Atkinson, A. et al. Krill (Euphausia superba) distribution contracts southward during rapid regional warming. Nature Climate Change 9, 142–147 (2019).
Murphy, E. J. et al. Interannual variability of the South Georgia marine ecosystem: biological and physical sources of variation in the abundance of krill. Fisheries Oceanography 7(3–4)), 381–390 (1998).
Reid, K. & Croxall, J. P. Environmental response of upper trophic-level predators reveals a system change in an Antarctic marine ecosystem. Proceedings of the Royal Society of London. Series B: Biological Sciences 268(1465), 377–384 (2001).
Waluda, C. M., Hill, S. L., Peat, H. J. & Trathan, P. N. Long-term variability in the diet and reproductive performance of penguins at Bird Island, South Georgia. Marine Biology 164(3), 39 (2017).
Forcada, J. & Hoffman, J. I. Climate change selects for heterozygosity in a declining fur seal population. Nature 511, 462–465 (2014).
Bengtson J. L., Ferm L. M., Härkönen T. J. & Stewart B. S. Abundance of Antarctic Fur Seals in the South Shetland Islands, Antarctica, During the 1986/87 Austral Summer in Antarctic Ecosystems (Eds Kerry, K. R. & Hempel, G.) 265–270 (Springer, 1990).
Hucke-Gaete, R., Osman, L. P., Moreno, C. A. & Torres, D. Examining natural population growth from near extinction: the case of the Antarctic fur seal at the South Shetlands, Antarctica. Polar Biology 27(5), 304–311 (2004).
Breed, G. A., Bowen, W. D., McMillan, J. I. & Leonard, M. L. Sexual segregation of seasonal foraging habitats in a non-migratory marine mammal. Proceedings of the Royal Society B: Biological Sciences 273(1599), 2319–2326 (2006).
Nicol, S., Foster, J. & Kawaguchi, S. The fishery for Antarctic krill – recent developments. Fish and Fisheries 13(1), 30–40 (2012).
Estrada, J. A., Rice, A. N., Natanson, L. J. & Skomal, G. B. Use of isotopic analysis of vertebrae in reconstructing ontogenetic feeding ecology in white sharks. Ecology 87(4), 829–834 (2006).
Drago, M. et al. Isotopic niche partitioning between two apex predators over time. Journal of Animal Ecology 86(4), 766–780 (2017).
Hill, S. L., Atkinson, A., Pakhomov, E. A. & Siegel, V. Evidence for a decline in the population density of Antarctic krill Euphausia superba still stands. A comment on Cox et al. Journal of Crustacean Biology 39(3), 316–322 (2019).
Hanson, N. N., Wurster, C. M., Bird, M. I., Reid, K. & Boyd, I. L. Intrinsic and extrinsic forcing in life histories: patterns of growth and stable isotopes in male Antarctic fur seal teeth. Marine Ecology Progress Series 388, 263–272 (2009).
Bergmann, K. G. L. C. Über die Verhältnisse der wärmeokönomie der Thiere zu ihrer Grösse. Göttinger Studien 3, 595–708 (1847).
Alonso, J. C., Salgado, I. & Palacín, C. Thermal tolerance may cause sexual segregation in sexually dimorphic species living in hot environments. Behavioral Ecology 27(3), 717–724 (2015).
Agashe, D. & Bolnick, D. I. Intraspecific genetic variation and competition interact to influence niche width. Proceedings of the Royal Society B: Biological Sciences 277, 2915–2924 (2010).
Cloyed, C. S. & Eason, P. K. Niche partitioning and the role of intraspecific niche variation in structuring a guild of generalist anurans. Royal Society open science 4(3), 170060 (2017).
Hawkes, L. A. et al. Phenotypically linked dichotomy in sea turtle foraging requires multiple conservation approaches. Current Biology 16(10), 990–995 (2006).
Sato, K. et al. Stroke frequency, but not swimming speed, is related to body size in free-ranging seabirds, pinnipeds and cetaceans. Proceedings of the Royal Society B: Biological Sciences 274(1609), 471–477 (2006).
Williams, T. M. The evolution of cost efficient swimming in marine mammals: limits to energetic optimization. Philosophical Transactions of the Royal Society B 354, 193–201 (1999).
Ficetola, G. F. & De Bernardi, F. Trade-off between larval development rate and post-metamorphic traits in the frog Rana latastei. Evolutionary Ecology 20(2), 143–158 (2006).
Warren, N. L., Trathan, P. N., Forcada, J., Fleming, A. & Jessopp, M. J. Distribution of post-weaning Antarctic fur seal Arctocephalus gazella pups at South Georgia. Polar Biology 29(3), 179–188 (2006).
Patrick, S. C., Pinaud, D. & Weimerskirch, H. Boldness predicts an individual’s position along an exploration–exploitation foraging trade‐off. Journal of Animal Ecology 86(5), 1257–1268 (2017).
Charnov, E. L. Optimal foraging, the marginal value theorem. Theoretical Population Biology 9, 129–136 (1976).
Araújo, M. S. et al. Network analysis reveals contrasting effects of intraspecific competition on individual vs. population diets. Ecology 89(7), 1981–1993 (2008).
Goubault, M., Outreman, Y., Poinsot, D. & Cortesero, A. M. Patch exploitation strategies of parasitic wasps under intraspecific competition. Behavioral Ecology 16(4), 693–701 (2005).
Casey, T. M. Energetics of locomotion. Advances in Comparative and Environmental Physiology 11, 251–275 (1992).
Atkinson, A. et al. Oceanic circumpolar habitats of Antarctic krill. Marine Ecology Progress Series 362, 1–23 (2008).
Green, K., Burton, H. R. & Williams, R. The diet of Antarctic fur seals Arctocephalus gazella (Peters) during the breeding season at Heard Island. Antarctic Science 1(4), 317–324 (1989).
Klages, N. T. W. & Bester, M. N. Fish prey of fur seals Arctocephalus spp. at subantarctic Marion Island. Marine Biology 131(3), 559–566 (1998).
Baker, J. R. & McCann, T. S. Pathology and bacteriology of adult male Antarctic fur seals, Arctocephalus gazella, dying at Bird Island, South Georgia. British Veterinary Journal 145(3), 263–275 (1989).
Paritte, J. M. & Kelly, J. F. Effect of cleaning regime on stable-isotope ratios of feathers in Japanese Quail (Coturnix japonica). The Auk 126(1), 165–174 (2009).
Qi, H., Coplen, T. B., Geilmann, H., Brand, W. A. & Böhlke, J. K. Two new organic reference materials for δ13C and δ15N measurements and a new value for the δ13C of NBS 22 oil. Rapid Communications in Mass Spectrometry 17(22), 2483–2487 (2003).
Coplen, T. B. et al. New guidelines for δ 13C measurements. Analytical Chemistry 78(7), 2439–2441 (2006).
Boyd, I. L. & Roberts, J. P. Tooth growth in male Antarctic fur seals (Arctocephalus gazella) from South Georgia: an indicator of long‐term growth history. Journal of Zoology 229(2), 177–190 (1993).
Beamish, R. J. & Fournier, D. A. A method for comparing the precision of a set of age determinations. Canadian Journal of Fisheries and Aquatic Sciences 38(8), 982–983 (1981).
Rösch, A. & Schmidbauer, H. WaveletComp 1.1. WaveletComp: Computational Wavelet Analysis. R package version 1.1. At https://CRAN.R-project.org/package=WaveletComp (Date accessed: 15-11-2019) (2018).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. At http://www.r-project.org/ (Date accessed: 15-11-2019) (2019).
Healy, K. et al. SIDER: An R package for predicting trophic discrimination factors of consumers based on their ecology and phylogenetic relatedness. Ecography 41(8), 1393–1400 (2018).
Jackson, A. L., Inger, R., Parnell, A. C. & Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER – Stable Isotope Bayesian Ellipses in R. Journal of Animal Ecology 80(3), 595–602 (2011).
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. At https://CRAN.R-project.org/package=nlme (Date accessed: 15-11-2019) (2019).
Araújo, M. S., Bolnick, D. I. & Layman, C. A. The ecological causes of individual specialisation. Ecology Letters 14(9), 948–958 (2011).
Roughgarden, J. Evolution of niche width. American Naturalist 106, 683–718 (1972).
Bolnick, D. I., Yang, L. H., Fordyce, J. A., Davis, J. M. & Svanbäck, R. Measuring individual‐level resource specialization. Ecology 83(10), 2936–2941 (2002).
Source: Ecology - nature.com


