Larson, G. et al. Current perspectives and the future of domestication studies. Proc. Natl Acad. Sci. USA 111, 6139–6146 (2014).
Zohary, D. & Hopf, M. Domestication of Plants in the Old World: the Origin and Spread of Cultivated Plants in West Asia, Europe and the Nile Valley (Oxford Univ. Press, 2000).
Zeder, M. A., Bradley, D. G., Smith, B. D. & Emshwiller, E. Documenting Domestication: New Genetic and Archaeological Paradigms (Univ. California Press, 2006).
Piperno, D. R. & Pearsall, D. M. The Origins of Agriculture in the Lowland Neotropics (Academic, 1998).
Piperno, D. R. The origins of plant cultivation and domestication in the New World tropics: patterns, process, and new developments. Curr. Anthropol. 52, S453–S470 (2011).
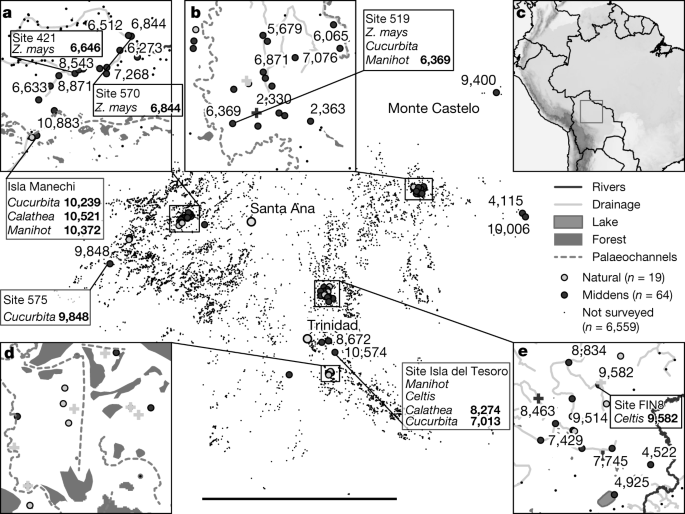
Clement, C. R., de Cristo-Araújo, M., d’Eeckenbrugge, G. C., Alves Pereira, A. & Picanço-Rodrigues, D. Origin and domestication of native Amazonian crops. Diversity (Basel) 2, 72–106 (2010).
Olsen, K. & Schaal, B. Microsatellite variation in cassava (Manihot esculenta, Euphorbiaceae) and its wild relatives: further evidence for a southern Amazonian origin of domestication. Am. J. Bot. 88, 131–142 (2001).
Sanjur, O. I., Piperno, D. R., Andres, T. C. & Wessel-Beaver, L. Phylogenetic relationships among domesticated and wild species of Cucurbita (Cucurbitaceae) inferred from a mitochondrial gene: implications for crop plant evolution and areas of origin. Proc. Natl Acad. Sci. USA 99, 535–540 (2002).
Clement, C. R. et al. The domestication of Amazonia before European conquest. Proc. R. Soc. Lond. B 282, 20150813 (2015).
Scaldaferro, M. A., Barboza, G. E. & Acosta, M. C. Evolutionary history of the chili pepper Capsicum baccatum L. (Solanaceae): domestication in South America and natural diversification in the seasonally dry tropical forests. Biol. J. Linn. Soc. 124, 466–478 (2018).
Watling, J. et al. Direct archaeological evidence for Southwestern Amazonia as an early plant domestication and food production centre. PLoS ONE 13, e0199868 (2018).
Lombardo, U. et al. Early and middle Holocene hunter-gatherer occupations in western Amazonia: the hidden shell middens. PLoS ONE 8, e72746 (2013).
Capriles, J. M. et al. Persistent Early to Middle Holocene tropical foraging in southwestern Amazonia. Sci. Adv. 5, eaav5449 (2019).
Hilbert, L. et al. Evidence for mid-Holocene rice domestication in the Americas. Nat. Ecol. Evol. 1, 1693–1698 (2017).
Lombardo, U. et al. Holocene land cover change in south-western Amazonia inferred from paleoflood archives. Global Planet. Change 174, 105–114 (2019).
Chandler-Ezell, K., Pearsall, D. M. & Zeidler, J. A. Root and tuber phytoliths and starch grains document manioc (Manihot esculenta) arrowroot (Maranta arundinacea) and llerén (Calathea sp.) at the Real Alto site, Ecuador. Econ. Bot. 60, 103–120 (2006).
Piperno, D. R. Phytoliths (AltaMira Press, 2006).
Morcote-Ríos, G., Bernal, R. & Raz, L. Phytoliths as a tool for archaeobotanical, palaeobotanical and palaeoecological studies in Amazonian palms. Bot. J. Linn. Soc. 182, 348–360 (2016).
Hanelt, P., Buttner, R. & Mansfeld, R. Mansfeld’s Encyclopedia of Agricultural and Horticultural Crops (except Ornamentals) (Springer, 2001).
Smith, B. D. The initial domestication of Cucurbita pepo in the Americas 10,000 years ago. Science 276, 932–934 (1997).
Piperno, D. R. & Stothert, K. E. Phytolith evidence for early Holocene Cucurbita domestication in southwest Ecuador. Science 299, 1054–1057 (2003).
Dillehay, T. D. & Piperno, D. R. in The Cambridge World Prehistory (eds Renfrew, C. & Bahn, P.) 970–985 (Cambridge Univ. Press, 2014).
Kistler, L. et al. Multiproxy evidence highlights a complex evolutionary legacy of maize in South America. Science 362, 1309–1313 (2018).
Rival, L. & McKey, D. Domestication and diversity in manioc (Manihot esculenta Crantz ssp. esculenta, Euphorbiaceae). Curr. Anthropol. 49, 1119–1128 (2008).
Rodrigues, L., Lombardo, U. & Veit, H. Design of pre-Columbian raised fields in the Llanos de Moxos, Bolivian Amazon: differential adaptations to the local environment? J. Archaeol. Sci. Rep. 17, 366–378 (2018).
McKey, D., Cavagnaro, T. R., Cliff, J. & Gleadow, R. J. C. Chemical ecology in coupled human and natural systems: people, manioc, multitrophic interactions and global change. Chemoecology 20, 109–133 (2010).
Jones, M. in The Evolution of Hominin Diets (eds Hublin, J.-J. & Richards, M. P.) 171–180 (Springer, 2009).
Aceituno, F. J. & Loaiza, N. The origins and early development of plant food production and farming in Colombian tropical forests. J. Anthropol. Archaeol. 49, 161–172 (2018).
Smith, B. D. General patterns of niche construction and the management of ‘wild’ plant and animal resources by small-scale pre-industrial societies. Phil. Trans. R. Soc. Lond. B 366, 836–848 (2011).
Lombardo, U., May, J.-H. & Veit, H. Mid- to late-Holocene fluvial activity behind pre-Columbian social complexity in the southwestern Amazon basin. Holocene 22, 1035–1045 (2012).
Manning, A. D., Fischer, J. & Lindenmayer, D. B. Scattered trees are keystone structures – implications for conservation. Biol. Conserv. 132, 311–321 (2006).
Tews, J. et al. Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J. Biogeogr. 31, 79–92 (2004).
Berkunsky, I. et al. Assessing the use of forest islands by parrot species in a Neotropical savanna. Avian Conserv. Ecol. 10, 11 (2015).
Prümers, H. & Jaimes Betancourt, C. 100 años de investigación arqueológica en los Llanos de Mojos. Arqueoantropológicas 4, 11–53 (2014).
Junqueira, A. B., Shepard, G. H. & Clement, C. R. J. E. B. Secondary forests on anthropogenic soils of the middle Madeira river: valuation, local knowledge, and landscape domestication in Brazilian Amazonia. Econ. Bot. 65, 85–99 (2011).
Lombardo, U., Canal-Beeby, E. & Veit, H. Eco-archaeological regions in the Bolivian Amazon: linking pre-Columbian earthworks and environmental diversity. Geogr. Helv. 66, 173–182 (2011).
Langstroth Plotkin, R. Biogeography of the Llanos de Moxos: natural and anthropogenic determinants. Geogr. Helv. 66, 183–192 (2011).
Lombardo, U., Denier, S. & Veit, H. Soil properties and pre-Columbian settlement patterns in the monumental mounds region of the Llanos de Moxos, Bolivian Amazon. Soil (Gottingen) 1, 65–81 (2015).
Rodrigues, L., Lombardo, U., Canal Beeby, E. & Veit, H. Linking soil properties and pre-Columbian agricultural strategies in the Bolivian lowlands: the case of raised fields in Exaltación. Quat. Int. 437, 143–155 (2017).
Boixadera, J., Poch, R. M., García-González, M. T. & Vizcayno, C. Hydromorphic and clay-related processes in soils from the Llanos de Moxos (northern Bolivia). Catena 54, 403–424 (2003).
Hanagarth, W. Acerca de la Geoecología de las Sabanas del Beni en el Noreste de Bolivia (Instituto de Ecología, 1993).
Lombardo, U., Ruiz-Pérez, J. & Madella, M. Sonication improves the efficiency, efficacy and safety of phytolith extraction. Rev. Palaeobot. Palynol. 235, 1–5 (2016).
Piperno, D. R. Identifying crop plants with phytoliths (and starch grains) in Central and South America: a review and an update of the evidence. Quat. Int. 193, 146–159 (2009).
Iriarte, J. Assessing the feasibility of identifying maize through the analysis of cross-shaped size and three-dimensional morphology of phytoliths in the grasslands of southeastern South America. J. Archaeol. Sci. 30, 1085–1094 (2003).
Watling, J. et al. Differentiation of Neotropical ecosystems by modern soil phytolith assemblages and its implications for palaeoenvironmental and archaeological reconstructions II: southwestern Amazonian forests. Rev. Palaeobot. Palynol. 226, 30–43 (2016).
Dickau, R. et al. Differentiation of Neotropical ecosystems by modern soil phytolith assemblages and its implications for palaeoenvironmental and archaeological reconstructions. Rev. Palaeobot. Palynol. 193, 15–37 (2013).
Hogg, A. G. et al. SHCal13 Southern Hemisphere calibration, 0–50,000 years cal BP. Radiocarbon 55, 1889–1903 (2013).
Bronk Ramsey, C. Bayesian analysis of radiocarbon dates. Radiocarbon 51, 337–360 (2009).
Piperno, D. R. et al. Phytoliths in Cucurbita and other Neotropical Cucurbitaceae and their occurrence in early Archaeological sites from the lowland American tropics. J. Arch. Sci. 27, 193–208 (2000).
Source: Ecology - nature.com


