Prevalence of E. coli and their antibiotic susceptibility
Escherichia coli prevalence (colony-forming unit (CFU) ml−1) was observed in the Kelani River from upstream (K1; Seethawaka Export Processing Zone [EPZ]) to downstream (K4; Ambathale intake) (Table 1). We did not observe the large change in the range of prevalence of total coliform in October (23–31 CFU ml−1) and March (17–26 CFU ml−1), but the maximum number of E. coli isolated in October (27 CFU ml−1) was three times higher than in March (n = 9 CFU ml−1). At the Seethawaka EPZ sampling point, a considerably higher E. coli prevalence was measured as compared to downstream sampling points, which probably suggests flushing of poorly treated industrial wastewater of Seethawaka EPZ. If we compared the maximum observed E. coli (CFU ml−1) of the Kelani River with some rivers in the emerging countries, the Kelani condition seems better than Chaophraya River (70 CFU ml−1), and Ping River (42 CFU ml−1), of Thailand, the Brahmaputra River (42 CFU ml−1), Sabarmati River (42 CFU ml−1), and the Ganges Rivers (42 CFU ml−1) of India and almost comparable with rivers like Nan (4.8 CFU ml−1), Wang (0.47 CFU ml−1), and Yom (5.4 CFU ml−1) in Thailand33.
We tested susceptibility and resistance of E. coli isolated from the samples for three fluoroquinolones, that is, norfloxacin (NFX), ciprofloxacin (CIP), and levofloxacin (LVX), and three non-fluoroquinolones, namely, kanamycin monosulfate (KM), tetracycline (TC), and sulfamethoxazole (ST). The resistance percentage of the fluoroquinolone (LVX, CIP, and NFX) decreased from upstream K1 to downstream K4 in the Kelani river (Fig. 2). This could be due to the self-purification of the river or the degradation of available antibiotics. Observed seasonal variation is hinting at the influences of climatic factors on the antibiotic susceptibility. Fluoroquinolones (LVX, CIP, NFX) showed a similar trend as their susceptibility also decreased in the summer season at all locations except the susceptibility for NFX at sampling location K4.
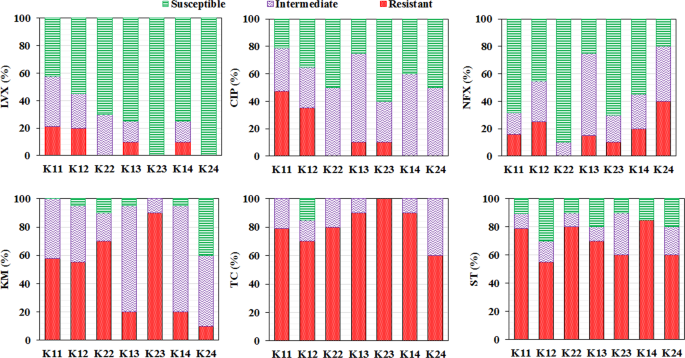
Bar diagram exhibiting percentage of different class of antibiotic resistance (i.e., resistant, intermediate, and susceptible). For a levofloxacin (LVX), b ciprofloxacin (CIP), c norfloxacin (NFX), d kanamycin monosulfate (KM), e tetracycline (TC), and f sulfamethoxazole (ST) in the Kelani River of 2017 representing wet season (K11–K14) and dry season (K22–K24) in 2018.
When compared to the Chaophraya River, Thailand, which was subjected to a similar kind of study, fluoroquinolones (LVX, CIP, and NFX) have demonstrated a higher resistance percentage along the urbanized area33. However, for the Chaophraya River, land-use patterns were considered as the governing factor to influence resistance for the fluoroquinolones, which seems not true for the Kelani River, which seems to have influenced by climatic factor, rains, enrichments, dilutions, WWTP, and DWTP like the presence of the WWTPs located upstream of the first (after Seethawala EPZ) and third sampling points (after biyagama EPZ). For non-fluoroquinolone (KM, ST, and TC) higher resistance was observed at downstream locations compared to upstream locations, which probably indicates the antibiotic use pattern affecting the resistance33. A thorough investigation is needed to further strengthen these results by adding more sampling locations at both the ends, that is, before the Seethawaka WWTP, and along with the urbanized downstream areas.
Antibiotic resistance genes
A higher concentration of ARG was found in more urbanized sites of Sri Lanka (Fig. 3). Two out of three genes studied (blaCTX1 and qnrs) were found to be considerably higher at all sites in the summer (March) season than in winter (October). This shows that there is a substantial impact of seasons on the prevalence of ARG as well. It may be attributed to a higher influx of ARG in the downstream during wet seasons with higher discharge. Genes that confer resistance to the old antibiotics, TCs (tet) and sulfonamides (sul), were detected in all samples (Table 2). The detection of sul1 in both seasons is following the antibiotic resistance test results where resistance to sulfonamides (i.e., ST) was observed. Sulfonamides are old antibiotics that have been extensively used in the past but are not used anymore for human consumption because of its toxicity. However, it is still used in agriculture, and the genes encoding resistance for these antibiotics were found to be persistent34,35. A study found that genes conferring resistance to fluoroquinolones (qnrS, aac(6′)-Ib-cr) were more likely to co-occur with ampC in a plasmid of Serratia marcescens36. Gene ampC gene, which confers resistance to β-lactam antibiotics (e.g., ampicillin) was detected in 2017 but not in 2018. Metals showed varied results among each other, yet higher EC and increased metals may be enhancing the occurrence of ARGs3. Apparent selecting pressure of antibiotics on ARG abundance is observed in the rivers of emerging countries like Pakistan37, China (Haihe River38, Beijiang River39), Thailand, and India40.
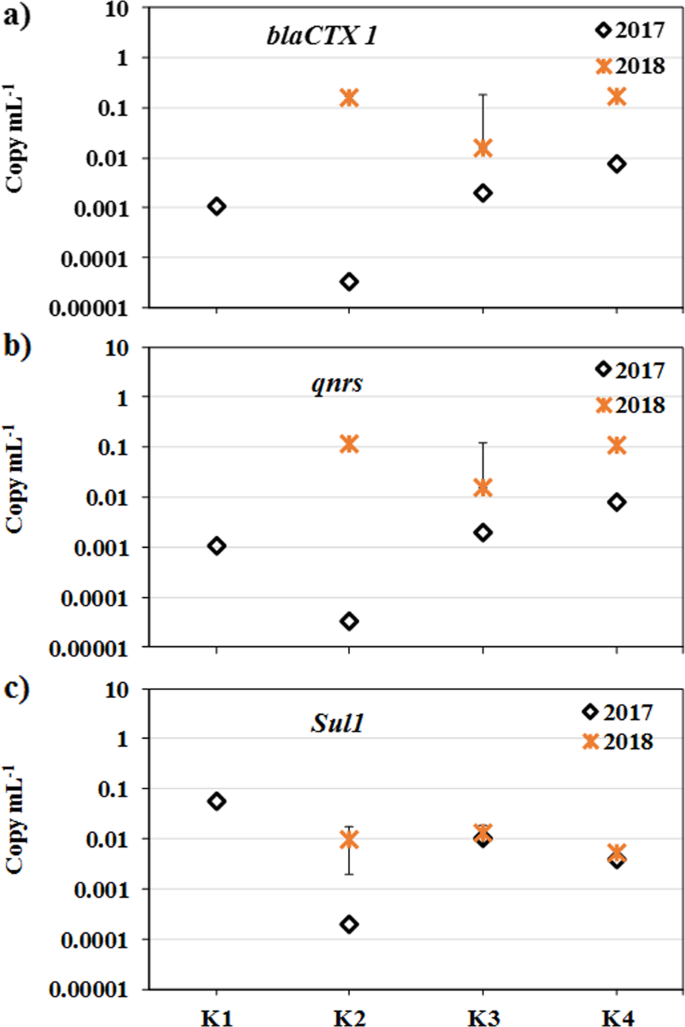
Scattered plot exhibiting ARG concentration for a blaCTX, b qnrS, and c sul1 in the Kelani River. (The major observation was that the number of copies in the dry season of March 2018 was higher than the wet season of October 2017. Error bars show the variation in terms of standard deviation using sample triplicate analysis).
Tracing the multi- and cross-resistance through source apportionment
In our study, variations among in situ parameters, metal, fecal coliform, ARB, and ARG, were explained by four principal components (PCs) explaining 84.74% of the total variance in the data (Supplementary Table 1) and presented as the scattered plot in Fig. 4a, b. The first component (PC_1) accounted for 26.84% variance and was comprised of pH, temperature, EC, ARG (blaCTX1 and qnrs), and ARB (LVX). The second component (PC_2), represented by metal (Mn), ARG (sul1), oxidation–reduction potential (ORP), and E. coli, showed a variance of 21.47%. While PC_3 included Zn, NFX, KM, and TC and explained variance of 19.08%, PC_4 showed higher loading for Pb and Cd, accounting for only 17.35% variance in the data. On the other hand, the cluster diagram illustrated that the number of components was four in October 2017, which decreased to three in March 2018 (Fig. 4c, d), implying the seasonality significance on the variability of antibiotic resistance.
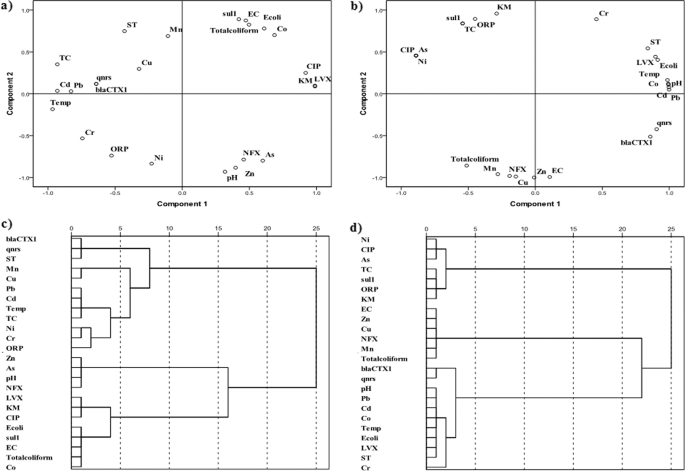
X–Y scatter plots of first two principal component loading for a 2017 and b 2018, and dendrogram representation of the result of cluster analyses for c 2017 and d 2018. The significant observation was that the governing factors and cluster decreased in the dry season of March with respect to the wet season in October 2018.
We made two distinct of observations from X–Y plot of PC_1 and PC_2: (i) metal association with antibiotic resistance was higher in October as evident from the positive–negative domain of PC_1 and PC_2 at left upper domain; and (ii) ARB and ARG parameters occupied all four domains of the plus created by loadings of PC_1 and PC_2 in March 2018, which were restricted to only positive loading domains of PC_1. This may further imply that during October, related antibiotic parameters were at the forefront governing greater variation in the river water. However, in the next 6 months, by the month of March, the water quality of the river might have become more consistent in terms of both fecal and antibiotic resistance contaminations. This is an interesting observation, which hints at the requirement of scientific discussions on causative and associative features of traditional water quality parameters with anti-microbial features of the given ambient water. This further relates to the need of identification of proper markers for ARB and ARG prevalence in the environment as it is quite evident that the prevalence of E. coli is not a valid marker as seen in several WTPs studies and pollution levels, that is, biological oxygen demand, dissolved oxygen (DO), metal, microplastic, salinity, or other can be predictive feature to a certain extent only.
Figure 5 summarizes the results of this study in the conceptual flow diagram. As evident, almost all the sampling locations on Kelani River, Sri Lanka contained E. coli strains that exhibited the resistance to more than one antibiotic. Among six antibiotics examined for possible resistance, TC and ST resulted in the highest resistance and considerable seasonal impact on the prevalence of ARB and ARGs in the river was observed. We observed an increase in the number of ARG copies during dry season, that is, March 2018 than that of the wet season, that is, October 2017. It implies that ARG prevalence and transport are not only source dependent but are also dependent on factors such as seasonality, discharge, hydrological processes like evaporation, rainfall, runoff, and combined sewer overflow, associated water quality parameters, disease types in a particular time, and accordingly prescribed antibiotic, as well as people’s perception, awareness, and attitude. However, the number of factors regulating the ARG transmission in the studied river system got reduced in March 2018 than that in October 2017. Close associations of both ARB and ARGs seem to be there with contaminant or ionic enrichment, leading to an increase in the overall electrical conductivities (ECs) of the river water in the dry season.
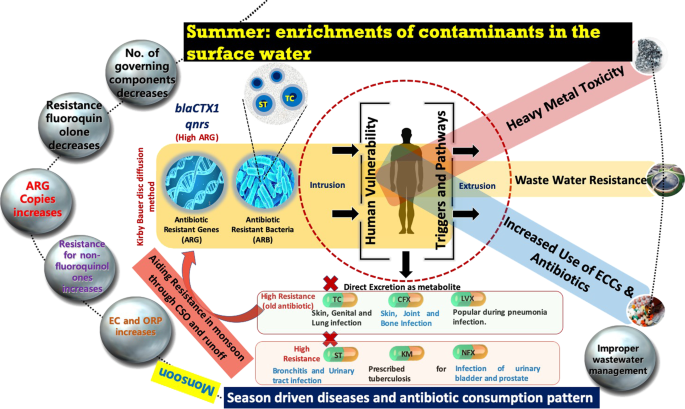
Conceptual model depiction of the result summary highlighting the influence of seasonality on antibiotic resistance.
Finally, we foresee an appalling need to reduce the infection mode of antibiotic resistance like best water and sanitation practices, awareness of cleanliness, more efficient and specific nature-based treatment solutions, and controlling the wastewater discharge to the surface water, especially rivers in the emerging countries are required to control the antibiotic concentration of surface waters. It is imperative to identify the available antibiotic concentrations to come up with a treatment mechanism. Further, ARBb and ARGs cannot be truly quantified by their concentrations in water columns, but the monitoring extension towards sediments is also required. This alternatively means that there is a need for implementation of a tertiary treatment with effective disinfection and management of sludge. Identification of the critical locations where higher antibiotic resistance E. coli can be found along with the current level of resistance toward the antibiotic categories is crucial. Last but not least, data related to seasonal variations in the ARB and ARG around the world is still scarce and needs to be substantiated from various parts of the world, especially from the emerging countries for better management and control of antibiotic resistance menace.
Source: Ecology - nature.com


