Study species
For the experiments, we used the laboratory-cultured Daphnia magna (clone V) originally provided by the Federal Environment Agency (Berlin, Germany). D. magna were cultured in a temperature conditioned room (20 ± 1 °C) with a light:dark photoperiod of 16:8 h. ASTM reconstituted hard freshwater36, additionally enriched with selenium and vitamins (biotin, thiamine hydrochloride, cyanocobalamin)37 served as culture medium. Once a week the culture medium was renewed and juveniles were removed three times a week to avoid high density12. Test animals were fed daily with the green algae Desmodesmus subspicatus at a carbon concentration of 0.2 mg C/D. magna/day. Algae were cultured with an appropriate culture medium38 in an air conditioned room (24 ± 1 °C) under a 16:8 h light:dark photoperiod. Before use, algae were centrifuged, culture medium discarded and algae pellets resuspended with ultrapure water.
Silver-nanoparticles (NM-300K)
In this study, we used NM-300K particles from the OECD Working Party on Manufactured Nanomaterials (WPMN) Sponsorship39 as AgNPs. The aqueous dispersion of NM-300K contained 10 w/w % silver and two stabilizing agents (4% each of Polyoxethylene Glycerol Trioleate and Polyoxyethylene (20) Sorbita mono-Laurat (Tween 20)) and had an average particle size of 15 nm39. The stability of NM-300K particles in ASTM medium (at equal concentrations as used in this study) shown by STEM analyses also performed at the University of Siegen (Germany) is documented by Hartmann et al.12 and Galhano et al.40. Based on these data, we can confirm that the reference material NM-300K is stable over 24 h (longest period without water exchange) and did not change in shape and size (analysed with the same material as used in this study). A S/TEM image of AgNPs (NM-300K) dispersed in ASTM medium, measured directly after the preparation of the stock solution is shown in Fig. S1.
To generate a homogenous suspension of AgNPs, the NM-300K stock vial was sonicated in an ultrasonic water bath for 10 minutes (Bransonic 221 ultrasonic cleaner, Branson Ultrasonic, USA) prior to use. A working stock with a nominal concentration of 50 mg/L was prepared in ASTM medium to set the test concentrations. As a matrix control, the AgNP-free stabilization agent NM-300K DIS was used. A dispersant stock solution was prepared accordingly. In this solvent with AgNP-free stabilization agent NM-300K DIS we diluted kairomones (see below) for Treatment Ib.
Preparation of kairomone stock medium
Kairomone stock medium (predator medium, PM) was prepared in accordance with Barbosa et al.27. In total, we used eight randomly selected adult wild-type zebrafish Danio rerio from West Aquarium GmbH (Bad Lauterberg, Germany) with a body length of about 40 mm and kept them in one 8 L glass tank filled with ASTM medium (without additional salts and vitamins) for 24 h in a temperature-controlled room (26 ± 1 °C) under a light-dark cycle of 14:10 hours. Fish were fed with 160 D. magna of varying sizes and ages one day before collecting the predator medium (PM). No extra fish flake food was given. After 24 h, when all D. magna were consumed by D. rerio, adult fish were returned to their home tank (80 ×40 ×35 cm³) and debris was allowed to settle down for 10 minutes before the medium, containing fish kairomones (predator medium) was directly used in the experiment. The predator medium (PM) was taken out from the glass tank with a 1 L glass beaker without any additional filtering. The freshly prepared PM was made every day under the same conditions as described above to ensure a high concentration of fish kairomones from D. rerio for the experiment. In their home tank, D. rerio was cultured in 112 L glass tanks (80 ×40 ×35 cm³) in groups of 100 animals with a sex ratio of 50:50 under a light-dark cycle of 14:10 hours and a water temperature of 26 ± 1 °C, a pH-value of 7–7.5 and a conductivity of 450 µS/cm. Water exchange (40%) took place two times a week. Water in the tank was aerated and filtered continuously. In their home tank, fish were fed daily in the morning with dry flake food (JBL GmbH & Co. KG, Germany), and additionally three times a week in the afternoon with brine shrimp Artemia salina.
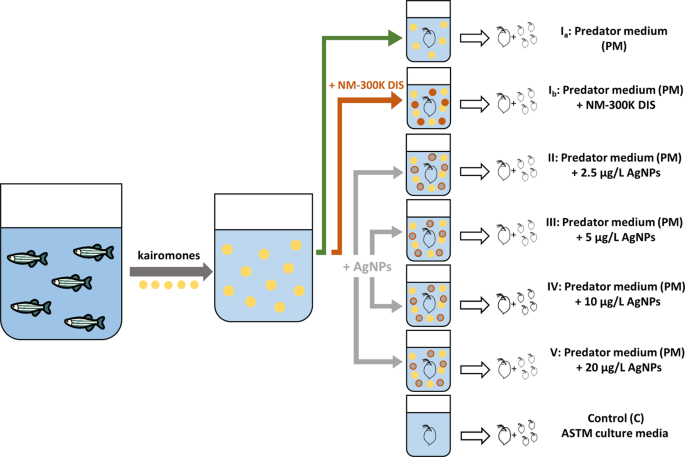
Experimental procedure and treatments
In this study, we followed the guidelines of the D. magna reproduction test14 and the method of Barbosa et al.27.. In all experiments, a single Daphnia (maternal generation) was placed in a glass beaker (100 mL, Rotilabo, Carl Roth GmbH + Co. KG, Karlsruhe), filled with 50 ml of test medium. Each D. magna was less than 24 h old at the start of the experiment. In each treatment group, maternal D. magna (n = 12) were exposed for 21 days. The offspring were removed from the test vessel as soon as possible and kept in ASTM medium without AgNPs. Thus, offspring were not exposed to AgNPs and we did not perform a multi-generational study. Medium renewal took place daily to ensure a high kairomone concentration throughout the complete test period. The O2 (mg/L), pH and temperature (°C) of old and fresh medium for one test beaker of each treatment group were measured once a week with a WTW Multi 3430 (WTW GmbH, Weilheim, Germany). Daphnia were fed daily with green algae Desmodesmus subspicatus with 0.2 mg C/D. magna/day algae suspension. We determined ‘time to first brood’, ‘reproduction’ (as the number of offspring), ‘maternal body length (mBL)’ (as distance from naupliar eye to the base of the dorsal spine) and ‘maternal spine length (mSL)’, and calculated ‘relative spine length of maternal Daphnia (mRSL)’ after each moult and after 21 days at the end of the experiment. We checked the beaker for offspring daily. We removed offspring of each brood from the beaker as soon as possible and measured ‘offspring body length (oBL)’, ‘offspring spine length (oSL)’, and ‘relative spine length of offspring (oRSL)’ as morphological traits. We took pictures of body length and spine length with a digital camera (Nikon Coolpix L830, Chiyoda, Tokyo, Japan) and analysed pictures using the software AxioVision (Carl Zeiss, Jena). We performed the following controls and treatments:
Ia. PM: Predator medium (PM) containing solely kairomones of D. rerio as a positive control for a kairomone induced response.
Ib. PM + NM-300K DIS: Predator medium (PM) enriched with NM-300K DIS as a control to exclude possible effects of the stabilization agent.
II. PM + 2.5 µg Ag/L: Predator medium (PM) enriched with 2.5 µg/L of AgNPs.
III. PM + 5 µg Ag/L: Predator medium (PM) enriched with 5 µg/L of AgNPs.
IV. PM + 10 µg Ag/L: Predator medium (PM) enriched with 10 µg/L of AgNPs.
V. PM + 20 µg Ag/L: Predator medium (PM) enriched with 20 µg/L of AgNPs.
C. Control: ASTM culture media as a reference.
In a previous study12, we investigated effects of AgNPs alone without kairomones on reproduction in D.magna using the same AgNP material and same AgNP-concentrations as used in this study. We detected a clear negative effect of AgNPs on the reproductive success of adult Daphnia over six generations. Based on the results of our former study we did not test the effects of AgNPs alone without kairomones here again. Exposure to NM-300K DIS alone, however, did not affect any morphological or life history traits in Daphnia12. Thus, we did not perform this additional control here.
All experiments were performed in accordance with relevant German guidelines and regulations.
Determination of total Ag in media samples
A single set (N = 1) of fresh and aged test media samples were collected during the 21-day test period to determine total Ag concentrations. The fresh media sample was taken on day 15 of the reproduction study and the aged media sample 24 h later (day 16), which represented the longest period without water exchange. The aqueous samples were stored at 4 °C prior to analysis. Total Ag content of the aqueous samples was determined with ICP-MS (inductively coupled plasma mass spectrometry; iCAP Qc, Thermo Fisher Scientific, Bremen, Germany). Prior to analysis, samples were taken out of the fridge and shaken for 30 minutes with a shaking machine (Edmund Bühler, Bodelshausen, Germany). The aqueous test samples were digested with concentrated nitric acid (70%, Analytical Reagent Grade, Fisher Scientific, Loughborough, UK) for 90 min and diluted 100 times to obtain a concentration of 2.9% (w/v) HNO3. Silver standard solution (Inorganic Ventures, Christiansburg, VA, USA) was used to calibrate the instrument on the same day, n = 10, 107Ag+ was measured, Indium (Inorganic Ventures, Christiansburg, VA, USA) served as an internal standard. All concentrations were calculated from calibration graphs using the internal standard correction. Limit of detection (LOD) and limit of quantification (LOQ) for 107Ag+ were 0.1 µg/L and 0.3 µg/L, respectively, depending on the experimental setup. The detailed experimental parameters are presented in Supplementary Table S1.
Statistical analysis
The statistical analysis was performed using the statistical program R version 3.2.441. For all parameters, we first compared parameters between maternal Daphnia from the control (ASTM medium, C) and from Treatment Ia (PM) to test whether D. rerio was a useful predator for testing anti-predator defence mechanism in maternal D. magna. Secondly, we analysed the differences between Treatment Ia (PM) and Treatments II – V (PM + different concentration of AgNPs), including Treatment Ib (PM + NM-300K DIS) to analyse the influence of PM in combination with AgNPs and to exclude possible effects of the dispersant agent on test animals (maternal Daphnia). For each treatment, we calculated the life-history parameters reproduction (cumulative mean number of offspring) ± standard deviation (sd), time to first brood (days ± sd), maternal body length (mBL; mm ± sd), offspring body length (oBL; mm ± sd), maternal spine length (mSL; mm ± sd), offspring spine length (oSL; mm ± sd), and checked the data for normal distribution (Shapiro-Wilk test) and for homogeneity of variances (Bartlett´s test). If both requirements met, we performed a one-way analysis of variances (ANOVA), followed by a Dunnett´s post hoc-test for multiple comparisons to test for statistical differences within treatments. Was one requirement not fulfilled, the nonparametric alternative, the Kruskal-Wallis test and afterwards the Dunn’s Test of multiple comparisons using rank sums42 was used. Because relative spine length of maternal Daphnia (mRSL) and relative spine length in offspring (oRSL) are bounded27, the data were analysed as dependent variables by using a ‘glmer’ (Generalized Linear Mixed Effect Model) of the package lme443. As fixed factor, we added treatment as the categorical variable to each model. Relative spine length of maternal Daphnia (mRSL) and relative spine length in offspring (oRSL) were modelled using a Gamma error distribution and a Log link function27. We included the number of moults and identity of test animals as nested random effects to the model. Model assumptions were checked visually. The p-values were adjusted with Bonferroni correction. Significant p-values were marked with asterisks (*P < 0.05, **P < 0.01, ***P < 0.001). All p-values are two tailed.
Source: Ecology - nature.com


