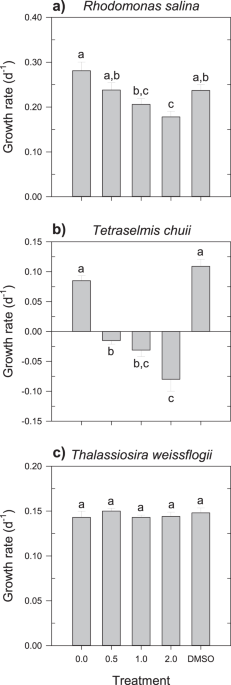
As expected, autotrophs were more resistant to rotenone, with the exception of T. chuii. Other species of the genus Tetraselmis are also reported to be more vulnerable to rotenone than other algae species, such as the marine Nannochloropsis oculata29 and the freshwater Chlorella kessleri30. This observation suggests that there may be a factor that is common to the genus Tetraselmis that enhances cellular susceptibility to this compound, although it is currently unknown. Conversely, the diatom and R. salina were quite resistant to rotenone effects. In the particular case of the cryptophyte, the effects of this compound were only evident during the exponential growth phase.
Cells undergo drastic metabolic changes when switching from exponential to stationary phases. For example, photosynthesis and respiration rates are, on average, higher during the exponential growth phase for auto- and mixotrophic species31. Similarly, heterotrophic ciliates and flagellates displayed higher respiration rates when actively growing than when stationary32. Therefore, it seems that the increased respiratory chain activity during the exponential phase enhances an organism’s susceptibility to rotenone. This conclusion aligns with the mechanism of action of rotenone, which, among other effects, is known to inhibit the synthesis of ATP26. A consequence of the reduced pool of available ATP can be seen in the assembly of microtubules, which becomes impaired and ultimately results in mitotic arrest and inhibition of cell proliferation33. Without these processes, the cell cannot divide, which would have a much higher impact on actively growing cells than on those progressing towards stationary phase.
The observed differences between growth phases under exposure to rotenone may have important consequences for the interpretation of laboratory and field experiments on single-celled organisms, not only with rotenone but with other toxic compounds as well. Despite having data exclusively from R. salina (which forces caution in the extrapolation of conclusions to other species), the results indicate that the effect of pollutants should always be tested using the same physiological conditions to minimise intra-specific differences. In the laboratory, this can be easily accomplished by controlling sampling times and/or by using a single batch of cultured organisms (as used in the experiments with mixo- and heterotrophs). On the other hand, for field work this may represent a challenge. Nonetheless, organisms in the field are likely living on an almost constant exponential growth phase (e.g. Dortch et al.34), diminishing the risk of comparisons.
A major result of the study is the high sensitivity of the heterotrophic ciliate S. arenicola to rotenone, in particular compared to the other heterotrophic predator tested, the dinoflagellate G. dominans. In fact, G. dominans, displayed a peculiar response to rotenone, showing negative growth rates and high ingestion rates at the lowest concentration, and similar growth rates and non-significant ingestion rates (two-tailed Student’s t-test, P = 0.114) at the highest. These results suggest that this dinoflagellate may be able to tolerate the presence of rotenone up to a concentration of 1.0 mg L−1 by maintaining key cellular processes (like phagocytosis) active while avoiding expensive ones such as cellular division. This could be a mechanism of survival that enables the endurance of harsh conditions for short time periods. Nonetheless, more data is needed to validate this hypothesis. On the other hand, planktonic ciliates are known to be highly susceptible to several chemical compounds, such as hydrocarbons and chemical dispersants35,36, but also to DMSO, although the toxicity of the latter is usually evidenced at higher concentrations than the ones used in this study37,38. Indeed, the ciliate S. arenicola was the only species whose growth was reduced by ca. 60% solely by the presence of DMSO.
Analogous to the results observed for heterotrophs, with the ciliate being more sensitive than the dinoflagellate, the mixotroph M. rubrum was more sensitive than K. armiger. In fact, the ingestion rate of M. rubrum was already negligible even with DMSO as the only added compound (Fig. 4a). Indeed, DMSO hindered the ingestion of prey for both ciliates (Fig. 4a,c). In this regard, a precursor of DMSO, β-dimethylsulfoniopropionate (DMSP), reduced the feeding of heterotrophic marine ciliates by 50–75%, whereas for heterotrophic dinoflagellates, this reduction was 28–40%39.
Overall, chloroplast-bearing predators displayed better resilience than heterotrophs at a concentration of 0.5 mg of rotenone L−1. However, their feeding rates were more affected, rendering the overall mixoplankton grazing impact on prey populations considerably lower than that of heterotrophic predators. In particular, K. armiger did not exhibit any evidence of feeding in the presence of rotenone (irrespective of concentration), while displaying only a slight negative growth rate in the highest concentration. These results are in agreement with those of previous studies on this species, in which it has been observed that K. armiger can survive long starvation periods using only chloroplasts for C acquisition, although barely dividing in the absence of food40, comparable to the non-significant ingestion rates observed in this study in the presence of rotenone.
One of the main motivations of this study was to test the effectiveness of the use of rotenone combined with the dilution technique to determine mixotrophic grazing. For the method to be useful, heterotrophic grazing should be impaired while leaving mixoplankton grazing unaffected. Despite the promising results for the effects of rotenone in terms of growth rates (with perhaps the caveat of the high sensitivity of T. chuii), the analysis of the effects of this compound on grazing highlighted severe limitations that were not predicted by the theoretical mechanism of action for rotenone (see Table 1). For instance, one of the assumptions of the study was that chloroplast-bearing organisms would be less affected by rotenone despite likely displaying a reduced ATP pool. A question that remains unanswered by this assumption is how large is the dependence of mixotrophic grazing processes on the ATP produced by the oxidative phosphorylation. In other words, can the photo phosphorylation supply enough ATP to maintain basal functions while enabling phagocytosis? The answer, at least from the present experiments, seems to be no, as explained next.
At 30 µE m−2 s−1 (the experimental conditions used in the present study), a well fed K. armiger fixes C at a higher rate than the maximum observed for unfed cells, and the chlorophyll a content is close to the maximum registered40. These observations suggest that this dinoflagellate maximises the use of the chloroplasts in situations akin to those used on the present study. Therefore, it seems plausible to assume that these conditions are less prone to magnify potential negative effects of rotenone on the behaviour of the CM.
For M. rubrum, it is known that the photosynthetic capacities depend on the quality of the chloroplasts acquired through the ingestion of cryptophytes from the genera Teleaulax, Plagioselmis or Geminigera41, and peak around 30 µE m−2 s−142. Ultimately, the sequestered chloroplasts require the presence of active cryptophyte nuclei, and start to lose photosynthetic efficiency after 3 days without the adequate food source43. Accordingly, during the exposure to rotenone, the chloroplasts of the NCM were also likely close to their full potential despite being fed with R. salina.
Contrary to all other tested predators, the heterotroph G. dominans was still able to ingest R. salina under concentrations of rotenone up to 1.0 mg L−1 (although with ca. 9% mortality and a grazing impact reduction, GIR, of ca. 83%). Indeed, it is important to note that with a concentration of 0.5 mg L−1, the absolute number of R. salina cells ingested per G. dominans was approximately 3.8× higher than that of K. armiger and 13.2× higher than that of M. rubrum in their respective control situations.
Thus, despite the fact that the chloroplasts of both mixotrophs tested here should have been in good conditions and that the available ATP pool for G. dominans was (likely) severely reduced during the exposure to rotenone, field grazing estimates using rotenone would be, at best, conservative. Indeed, the analysis of the GIR suggests that in a hypothetical dilution setting with rotenone (0.5 mg L−1) and all 3 predators, G. dominans would still be the major grazer. Hence, mixotrophic grazing is clearly affected by the reduced ATP concentration, although future physiological studies are required to elucidate the actual contribution of the oxidative phosphorylation for mixoplankton and its role on phagotrophy.
An example that further corroborates that heterotrophic dinoflagellates can display a substantial grazing impact on natural populations (thus further complicating the use of a heterotrophic grazing deterrent such as rotenone) is the fact that some areas of the Mediterranean Sea possess a biomass of heterotrophic dinoflagellates approximately 4× higher than that of autotrophic (with high potential for mixotrophy3) and mixotrophic species combined (not in a bloom situation)44. Additionally, the average C-specific ingestion rate of heterotrophic dinoflagellates is ca. 4.5× higher than that of their mixoplankton counterparts (see Fig. 10 in Calbet et al.45 and references therein), meaning that one can assume that heterotrophs would impact prey nearly 20× more than mixoplankton. Assuming that G. dominans is a good representative of heterotrophic dinoflagellates46, the presence of 0.5 mg L−1 would still render their impact (see Table 1) on prey populations ca. 11× higher than that of mixoplankton, which would be virtually zero (as per K. armiger results). Thus, rotenone cannot be used as an addition to the standard dilution technique for the purpose of deterring heterotrophic predation.
On an ecological note, rotenone has been used for decades to kill undesirable fish species in situ, and typical concentrations varied between 0.5 and 5.0 mg L−1, depending on the sensitivity of the target species47. However, as evidenced by the results of this study, considerably lower concentrations cause nefarious or even lethal effects on several planktonic species of distinct taxonomic groups. Additionally, the half-life of rotenone in aquatic environments ranges from hours to weeks48 and depends on several factors, namely temperature and pH, increases in which quicken degradation28. Hence, this information, together with the data gathered in this study for protists, and previous studies on zooplankton49,50 and rotifers29,30 suggest that the indiscriminate use of this compound in the past may have had disastrous consequences for aquatic food webs, whose extent is largely unknown.
Despite the inability of acting as a deterrent of heterotrophic grazing, rotenone can still be used as a good algal crop protector, especially if the predator is a sensitive organism like a ciliate (present study) or a rotifer29,30. Nevertheless, future measures should always assess the effect of rotenone on the specific organism that is plaguing the algal culture, as differences in the sensitivity towards the compound are expected, as seen in the present study. Similarly, the sensitivity of the algal culture itself should also be acknowledged before the application of rotenone, and factors such as the growth phase may be exploited to minimise the nefarious effects in non-target organisms.
Source: Ecology - nature.com


