Inoculum origin determines the operational niche of ethanol chain-elongating communities
Fifteen enriched cultures able to perform chain elongation with ethanol as electron donor were obtained from 6 sources (3 full-scale AD plant, 2 types of animal faeces, and 1 lab-scale pilot installation fed on thin stillage). Under the applied conditions – ethanol and acetate as sole electron donors/acceptors, low sulfate concentrations (0.2 mM) and BES addition to inhibit methanogenesis – chain elongation is the only known thermodynamically feasible metabolism for microbial growth30. These selective conditions allowed for a clear investigation on the effect of pH and source of inoculum on the features of chain-elongating microorganisms. The 3 AD-inocula could be enriched successfully at both neutral and mildly acidic pH. On the contrary, samples from animal faeces could only be enriched at neutral pH, while the inoculum from the thin stillage fermenter (pH 5.5) could only be enriched at pH 5.5. These pass-fail results show ethanol-based chain elongators are widely present, but the environment dictates the operational niche of the chain elongators. The pH of the intestinal tract of goat and sheep are close to neutrality, with a pH of 6.2–6.331. This environment could select against micro-organisms that are able to thrive during batch cultivation under mildly acidic conditions (pH 5.5), as performed in this study. Similarly, the thin stillage fermenter was run in CSTR mode, selecting for micro-organisms tolerant to the combination of low pH and caproic acid. Interestingly, these organisms were not able to cope with more lenient conditions at higher pH. Lastly, chain elongators could be enriched at neutral and mildly acidic pH both from the brewery AD granules (Lindemans, Van Steenberghe) and CSTR sludge AD plant. It is well known that within anaerobic digestion granules, gradients in pH exist32. This, in combination with varying feedstock compositions, could either create spatio-temporal niches in anaerobic digesters for chain elongators tolerating lower or higher pH, or select for a chain elongating community that has a broader pH range for growth. However, with the current information – amplicon sequencing and functional data – it is not possible to discern between these two hypotheses. Although this is outside the scope of this study, further investigating these hypotheses could teach us more about the ecological niche of C. kluyveri and how it survives and thrives in natural and engineered systems.
The enrichment of one C. kluyveri-related OTU that exhibits differential pH preferences suggests that different, currently undescribed C. kluyveri strains (or other, closely related species) with varying pH-preferences may have been enriched from the different inocula. To date, two strains of C. kluyveri have been isolated, with different functional pH ranges: type-strain DSM555 grows between pH 6 and 7.5 with optimum growth at 6.8, strain 3231B grows between pH 4.9 and 9.1, with optimal growth at pH between 6.4 and 7.622,23. The hypothesis that different C. kluyveri strains have different optimal pH and different pH ranges for growth, and are bound to specific pH-influenced niches in the environment could only be confirmed through further research, for instance through isolation of these strains, whole-genome sequencing, etc. The fact that some inocula could only be enriched at one pH indicates that, at least in those cases, this pH window for growth may be narrower than previously observed. Lastly, it should be mentioned that C. kluyveri was detected in only one of the six inocula (Go, 0.46% of the community, Supplementary Information, Section S.4). It is highly likely that concentrations in the other inocula were below the detection limit of Illumina-sequencing (0.003–0.01% in the analysed samples). C. kluyveri 3231B was isolated from bovine rumen, where the initial concentration of C. kluyveri was determined to be 0.00002–0.0002% of the ruminal community23. However, as mentioned before, under the imposed, highly restrictive conditions in this study, ethanol chain elongation is the only feasible metabolism, allowing for such high levels of enrichment.
pH determines product yield and community structure, but not growth rates
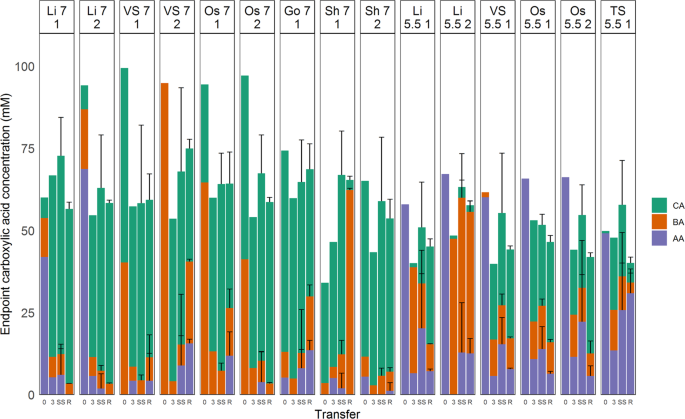
Final product concentrations of each enrichment after 12 transfers showed that pH was the key determining parameter affecting caproic acid production, with concentrations in enrichments at pH 5.5 being on average less than half of those at pH 7. The lower concentrations at pH 5.5, with same initial substrate concentrations, indicate toxicity prevented further accumulation, although toxicity of acetic and butyric acids could also play a role. At pH 7 acetate was usually depleted, implying it is likely higher concentrations of caproic acid could have been produced by these communities, if substrate availability had not been limiting. Despite the same substrate provided, the lower final concentrations at pH 5.5 seem to indicate that product toxicity or pH inhibition prevented further increases in product concentration. Although this was not tested in this study, caproic acid is known to be toxic to bacteria at all pH, although lower pH increase this effect19,28. While this study was restricted to batch growth of caproic acid-producing communities, in situ product extraction could alleviate product toxicity and allow the same conversion efficiency for communities at pH 5.5 and pH 7.
Both flow cytometric and phylogenetic characterisation of the enriched communities showed a clear distinction between communities at pH 5.5 and pH 7. While differences in flow cytometric fingerprints cannot be traced back to specific community shifts, genotypic characterisation showed that variation between communities was much lower at pH 5.5 than at pH 7, specifically due to the higher degree of enrichment of an OTU identifying closely with C. kluyveri. Several hypotheses can be proposed as to why the satellite community is larger at pH 7. Firstly, the combination of mildly acidic pH with relatively high concentrations of caproic acid creates a harsh environment, that suppresses non-tolerant organisms. At pH 7 on the other hand, the environment is less harsh, leaving more space for a satellite community to thrive. An alternative hypothesis is that the satellite community is reliant on necrotrophic growth, i.e. growth of bacteria on dead cell material. Communities were transferred after 7 days, rather than at the end of their exponential growth, and growth curve experiments showed that exponential phase was often over well before the 7-day mark. Cultures grown at neutral pH achieved higher optical densities at the end of the exponential phase, providing necrotrophs with more substrate during the stationary phase (Supplementary Information, Fig. S.12). Additionally, the presence of these satellite communities could be methodological artefacts, due to sampling after 7 days of incubation. Initially lenient conditions could allow a satellite community to flourish, while conditions subsequently become more stringent as caproic acid producers start to grow. Additionally, the length of the stationary phase might lead to changes in relative abundance of caproic acid producers and the satellite community, although predicting how this change influences the community would be tenuous. Ultimately, this could lead to the larger satellite community observed at pH 7. In the end, it seems likely that both hypotheses contribute in some way, with pH and caproic acid toxicity being the overarching drivers for the observed differences in satellite community.
Despite these stark differences in product output and community composition, growth rates were relatively similar across enrichments, ranging from 0.04 to 0.12 h−1. Given the lower caproic acid production observed at pH 5.5 it was anticipated that cultures at that pH would display significantly lower growth rates than their pH 7 counterparts, however, no significant differences in growth rates could be detected between communities grown at pH 5.5 and pH 7 (Fig. 2). These similar growth rates in most enrichments indicate that energy generation is not affected by pH, and it is likely product toxicity that prevents reaching higher concentrations of caproic acid and higher biomass densities. This in turn implies that efficient in situ product extraction technologies could allow for similar production rates at mildly acidic pH vs. circumneutral pH. Operation at lower pH had the additional advantage that it can inhibit methanogenic activity in the system without the need for expensive chemical inhibition. Lastly, we also demonstrated that lower pH selects for a simpler and more specialised community, minimising carbon losses to non-chain elongating community members. This set of traits – cheaper, more efficient operation with no loss of productivity – makes ethanol chain elongation at mildly acidic pH very attractive for future applications.
Limitations, shortcomings and prospects
This study demonstrates it is possible to enrich ethanol chain-elongation communities from various environments following a simple and straightforward methodology. All inocula selected in this study contained at least some chain elongating microorganisms, despite not being initially detected by the sequencing method applied here. These enriched communities were further characterised and investigated, but some attention should be given to potential pitfalls of the methodology applied.
All obtained enrichments harboured an OTU closely linked to C. kluyveri, in varying degrees of enrichment (9–99% relative abundance). The medium used for enrichment was a minimal medium derived from the DSM52 medium for growth of type strain C. kluyveri DSM555. This choice might have created a bias towards C. kluyveri-centered communities. Other ethanol-consuming chain elongators might have been present in the inocula, but not enriched due to lack of certain nutrients or trace elements in the medium, or, the used batch-culture approach selected for fast-growing organisms, rather than organisms with high substrate affinity but lower growth rates (i.e. r-strategists vs K-strategists33). Furthermore, the inoculum selection is by no means exhaustive, and (other) ethanol-consuming chain elongators can be present in other ecological niches. The initial type strain of C. kluyveri was isolated from canal mud10 and a latter strain obtained from cow rumen23. Recently, Kucek and co-workers reported ethanol-chain elongation communities grown on other synthetic media – with yeast extract – to be dominated by Acinetobacter and Rhodocyclaceae, not previously associated with ethanol-based chain elongation7. Given the above, this study is limited to the ecology of C. kluyveri-related chain elongators in the 4 engineered and 2 natural systems investigated. Despite this limitation, a distinct variation in pH differentiation was observed between the C. kluyveri communities present in these systems that was previously undescribed.
The obtained communities were stored after 12 generations, and later revived for the determination of their growth rates and genotypic community characterisation. It was observed that community structure was – in some cases – impacted by the storage, while in others, it remained unaffected. Several major trends could be observed in the shifts after storage: (i) it was no longer possible to clearly discern communities growing at pH 5.5 from those growing at pH 7 (Supplementary Information, Fig. S.3), (ii) some cultures became more enriched in the C. kluyveri-related OTU, and (iii) some other cultures became more enriched in an OTU related to Desulfovibrio (Fig. 3). The presence of this Desulfovibrio-OTU was unexpected, due to the low concentrations of sulfate in the medium (0.8 mM). However Desulfovibrio has been detected in bio-electrochemical systems where degradation of BES was observed34. While this study did not quantify BES degradation, it is possible this same process occurred. In some communities where Desulfovibrio became more abundant, a shift in product profile from caproic acid to butyric acid was observed. Specifically, it was observed that at pH 5.5, the presence of Desulfovibrio (i.e. relative abundance larger than 5%) significantly affected the butyric acid concentration (p < 0.001), while a similar observation at pH 7 was not possible due to the data not being distributed normally (Supplementary Information, Section S.6). A hypothesis for the effect of Desulfovibrio on the product profile could be the consumption of ethanol, or consumption of H2, which in turn can stimulate anaerobic ethanol oxidation, both possibilities lowering ethanol availability and shifting the product profile towards shorter acids. Although outside the scope of this study, variations between enrichments, microbial community structure and, consequently, the impact of storage could also be influenced by phage presence35. This indicates storage and preservation of mixed microbial communities can strongly impact community structure and composition, and care should be taken when doing so. For instance, in previous studies with a pure culture of C. kluyveri, researchers removed sulfate from the medium and substituted cysteine as sulfur source to avoid intrusion of sulfate reducing bacteria15. Similarly, the stability of enriched communities during storage could be improved by storing and reviving communities in conditions devoid of sulfate, instead using cysteine. Despite this undesired effect, many cultures preserved their caproic acid-producing capabilities, at the same pre-storage levels.
Another note to make is the lack of information on temporal changes in the product profile and community. In the previous section, temporal variation in conditions were hypothesised to impact community composition, for instance through necrotrophic growth or differential growth patterns. However, growth curves (Supplementary Material, Fig. S.12) could be used as a proxy for temporal changes. There it can be observed that the time when stationary phase is reached is highly variable between enrichments. At the same time, there is no clear link between when the stationary phase is reached and community composition or biomass concentration – e.g. higher initial biomass (as OD). However, OD is not an ideal reflection of cell concentrations, and the effect of, for instance, Desulfovibrio on growth of C. kluyveri could only be thoroughly elucidated by co-culture experiments.
Ultimately, this study demonstrates the presence of ethanol chain elongating organisms in a broad range of natural and engineered environments. The observation that ethanol chain elongating organisms are native to the sampled environments shows that these organisms can (at least) survive in these environments, if not thrive in them. Ethanol chain-elongators likely occupy a specific niche in these systems that they can exploit even at very low abundances, despite the high level of competition for substrates in most of these systems. Little to no information is available on the environmental niches of these organisms, and how they survive in a natural environment, while their widespread presence implies some role in environmental processes. At a bioprocess level, the results of this study show that the choice of inoculum should be made based on the desired process parameters. An environment that harbours only neutrophilic ethanol chain elongating organisms will not be suitable as a source of inoculum for a process where e.g. in-situ solvent-based extraction demands a mildly acidic operational pH.
Source: Ecology - nature.com


