Lieth, H. Modeling the Primary Productivity of the World. In Primary Productivity of the Biosphere (eds. Lieth, H. & Whittaker, R. H.) 237–263 (Springer Berlin Heidelberg). https://doi.org/10.1007/978-3-642-80913-2_12 (1975).
Gaston, K. J. Global patterns in biodiversity. Nature 405, 220–227 (2000).
Altizer, S. et al. Seasonality and the dynamics of infectious diseases: Seasonality and infectious diseases. Ecol. Lett. 9, 467–484 (2006).
Lisovski, S., Hoye, B. J. & Klaassen, M. Geographic variation in seasonality and its influence on the dynamics of an infectious disease. Oikos 126, 931–936 (2017).
Little, R. M. & Earlé, R. A. Sandgrouse (pterocleidae) and sociable weavers Philetarius socius lack avian haematozoa in semi-arid regions of South Africa. J. Arid Environ. 30, 367–370 (1995).
Valera, F., Carrillo, C. M., Barbosa, A. & Moreno, E. Low prevalence of haematozoa in Trumpeter finches Bucanetes githagineus from south-eastern Spain: additional support for a restricted distribution of blood parasites in arid lands. J. Arid Environ. 55, 209–213 (2003).
Guernier, V., Hochberg, M. E. & Guégan, J.-F. Ecology Drives the Worldwide Distribution of Human Diseases. PLOS Biol. 2, e141 (2004).
Piersma, T. Do Global Patterns of Habitat Use and Migration Strategies Co-Evolve with Relative Investments in Immunocompetence due to Spatial Variation in Parasite Pressure? Oikos 80, 623 (1997).
Mendes, L., Piersma, T., Lecoq, M., Spaans, B. & Ricklefs, R. E. Disease-Limited Distributions? Contrasts in the Prevalence of Avian Malaria in Shorebird Species Using Marine and Freshwater Habitats. Oikos 109, 396–404 (2005).
O’Connor, E. A., Cornwallis, C. K., Hasselquist, D., Nilsson, J.-Å. & Westerdahl, H. The evolution of immunity in relation to colonization and migration. Nat. Ecol. Evol. 1 (2018) https://doi.org/10.1038/s41559-018-0509-3.
Hosseini, P. R., Dhondt, A. A. & Dobson, A. Seasonality and wildlife disease: how seasonal birth, aggregation and variation in immunity affect the dynamics of Mycoplasma gallisepticum in house finches. Proc. R. Soc. Lond. B Biol. Sci. 271, 2569–2577 (2004).
Lowen, A. C., Mubareka, S., Steel, J. & Palese, P. Influenza Virus Transmission Is Dependent on Relative Humidity and Temperature. PLOS Pathog. 3, e151 (2007).
Metcalf, C. J. E. Invisible Trade-offs: Van Noordwijk and de Jong and Life-History Evolution. Am. Nat. 187, iii–v (2016).
Janeway, C. A. et al. Immunobiology. (Garland Science) (2001).
Horrocks, N. P. C., Matson, K. D. & Tieleman, B. I. Pathogen Pressure Puts Immune Defense into Perspective. Integr. Comp. Biol. 51, 563–576 (2011).
Lochmiller, R. L., Vestey, M. R. & Boren, J. C. Relationship between protein nutritional status and immunocompetence in northern bobwhite chicks. The Auk 503–510 (1993).
Nunn, C. L., Altizer, S. M., Sechrest, W. & Cunningham, A. A. Latitudinal gradients of parasite species richness in primates: Latitude and parasite species richness. Divers. Distrib. 11, 249–256 (2005).
Keesing, F., Holt, R. D. & Ostfeld, R. S. Effects of species diversity on disease risk. Ecol. Lett. 9, 485–498 (2006).
Salkeld, D. J., Trivedi, M. & Schwarzkopf, L. Parasite loads are higher in the tropics: temperate to tropical variation in a single host-parasite system. Ecography 31, 538–544 (2008).
Møller, A. P. Evidence of Larger Impact of Parasites on Hosts in the Tropics: Investment in Immune Function within and outside the Tropics. Oikos 82, 265–270 (1998).
Ndithia, H. K., Bakari, S. N., Matson, K. D., Muchai, M. & Tieleman, B. I. Geographical and temporal variation in environmental conditions affects nestling growth but not immune function in a year-round breeding equatorial lark. Front. Zool. 14 (2017).
Lafferty, K. D. The ecology of climate change and infectious diseases. Ecology 90, 888–900 (2009).
Sheldon, B. C. & Verhulst, S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321 (1996).
Klasing, K. C. Nutritional modulation of resistance to infectious diseases. Poult. Sci. 77, 1119–1125 (1998).
Ricklefs, R. E. & Wikelski, M. The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468 (2002).
Versteegh, M. A., Helm, B., Kleynhans, E. J., Gwinner, E. & Tieleman, B. I. Genetic and phenotypically flexible components of seasonal variation in immune function. J. Exp. Biol. 217, 1510–1518 (2014).
Mangino, M., Roederer, M., Beddall, M. H., Nestle, F. O. & Spector, T. D. Innate and adaptive immune traits are differentially affected by genetic and environmental factors. Nat. Commun. 8 (2017).
Helm, B. & Gwinner, E. Timing of Postjuvenal Molt in African (Saxicola torquata axillaris) and European (Saxicola torquata rubicola) Stonechats: Effects of Genetic and Environmental Factors. The Auk 116, 589–603 (1999).
Horrocks, N. P. C. et al. Immune Indexes of Larks from Desert and Temperate Regions Show Weak Associations with Life History but Stronger Links to Environmental Variation in Microbial Abundance. Physiol. Biochem. Zool. 85, 504–515 (2012).
Horrocks, N. P. C. et al. Environmental proxies of antigen exposure explain variation in immune investment better than indices of pace of life. Oecologia 177, 281–290 (2015).
Nelson, R. J., Demas, G. E., Klein, S. L. & Kriegsfeld, L. J. Seasonal Patterns of Stress, Immune Function, and Disease. (Cambridge University Press (2002).
Hegemann, A., Matson, K. D., Both, C. & Tieleman, B. I. Immune function in a free-living bird varies over the annual cycle, but seasonal patterns differ between years. Oecologia 170, 605–618 (2012).
Elgood, J. H., Sharland, R. E. & Ward, P. Palaearctic Migrants in Nigeria. Ibis 108, 84–116 (1966).
Elgood, J. H., Fry, C. H. & Dowsett, R. J. African Migrants in Nigeria. Ibis 115, 1–45 (1973).
Nwaogu, C. J. & Cresswell, W. Body reserves in intra-African migrants. J. Ornithol. 157, 125–135 (2016).
Crowe, T. M., Rebelo, A. G., Lawson, W. J. & Manson, A. J. Patterns of Variation in Body-Mass of the Black-Eyed Bulbul Pycnonotus Barbatus. Ibis 123, 336–345 (1981).
Nwaogu, C. J., Tieleman, B. I., Bitrus, K. & Cresswell, W. Temperature and aridity determine body size conformity to Bergmann’s rule independent of latitudinal differences in a tropical environment. J. Ornithol. 159, 1053–1062 (2018).
Nwaogu, C. J., Tieleman, B. I. & Cresswell, W. Weak breeding seasonality of a songbird in a seasonally arid tropical environment arises from individual flexibility and strongly seasonal moult. Ibis 161, 533–545 (2019).
Milla, A., Doumandji, S., Voisin, J.-F. & Baziz, B. Régime alimentaire du bulbul des jardins Pycnonotus barbatus (aves, pycnonotidae) dans le Sahel Algérois (Algérie). 60, 12 (2005).
Okosodo, E. F., Obasogie, F. O. & Orimaye, J. O. Food and Feeding Ecology of Common Bulbul (Pycnonotus barbatus) in Leventis Foundation Agricultural School Ilesa South Western Nigeria. Greener J. Agric. Sci. 6, 010–016 (2016).
Matson, K. D., Cohen, A. A., Klasing, K. C., Ricklefs, R. E. & Scheuerlein, A. No simple answers for ecological immunology: relationships among immune indices at the individual level break down at the species level in waterfowl. Proc. R. Soc. B Biol. Sci. 273, 815–822 (2006).
Versteegh, M. A., Schwabl, I., Jaquier, S. & Tieleman, B. I. Do immunological, endocrine and metabolic traits fall on a single Pace-of-Life axis? Covariation and constraints among physiological systems. J. Evol. Biol. 25, 1864–1876 (2012).
Nwaogu, C. J., Cresswell, W., Versteegh, M. A. & Tieleman, B. I. Seasonal differences in baseline innate immune function are better explained by environment than annual cycle stage in a year-round breeding tropical songbird. J. Anim. Ecol. 88, 537–553 (2019).
Tieleman, B. I., Williams, J. B., Ricklefs, R. E. & Klasing, K. C. Constitutive innate immunity is a component of the pace-of-life syndrome in tropical birds. Proc. R. Soc. B Biol. Sci. 272, 1715–1720 (2005).
Matson, K. D., Ricklefs, R. E. & Klasing, K. C. A hemolysis–hemagglutination assay for characterizing constitutive innate humoral immunity in wild and domestic birds. Dev. Comp. Immunol. 29, 275–286 (2005).
Adamo, S. How should behavioral ecologists interpret measures of immunity? vol. 68 (2004).
Pascual, M., Bouma, M. J. & Dobson, A. P. Cholera and climate: revisiting the quantitative evidence. Microbes Infect. 4, 237–245 (2002).
Young, B. E., Garvin, M. C. & McDonald, D. B. Blood parasites in birds from Monteverde, Costa Rica. J. Wildl. Dis. 29, 555–560 (1993).
Forbes, K. M. et al. Food limitation constrains host immune responses to nematode infections. Biol. Lett. 12, 20160471 (2016).
Nwaogu, C. J., Galema, A., Cresswell, W., Dietz, M. W. & Tieleman, B. I. A fruit diet rather than invertebrate diet maintains a robust innate immunity in an omnivorous tropical songbird. J. Anim. Ecol. https://doi.org/10.1111/1365-2656.13152 (2019).
Xu, D.-L., Hu, X.-K. & Tian, Y.-F. Effect of temperature and food restriction on immune function in striped hamsters (Cricetulus barabensis). J. Exp. Biol. 220, 2187–2195 (2017).
Martonne, E. de. Une Nouvelle fonction climatologique: L’Indice d’aridité. (Impr. Gauthier-Villars (1926).
Horrocks, N. P. C., Irene Tieleman, B. & Matson, K. D. A simple assay for measurement of ovotransferrin – a marker of inflammation and infection in birds: Ovotransferrin assay for plasma from wild birds. Methods Ecol. Evol. 2, 518–526 (2011).
Jain, S., Gautam, V. & Naseem, S. Acute-phase proteins: As diagnostic tool. J. Pharm. Bioallied Sci. 3, 118–127 (2011).
Matson, K. D., Horrocks, N. P. C., Versteegh, M. A. & Tieleman, B. I. Baseline haptoglobin concentrations are repeatable and predictive of certain aspects of a subsequent experimentally induced inflammatory response. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 162, 7–15 (2012).
Hegemann, A., Matson, K. D., Versteegh, M. A., Villegas, A. & Tieleman, B. I. Immune response to an endotoxin challenge involves multiple immune parameters and is consistent among the annual-cycle stages of a free-living temperate zone bird. J. Exp. Biol. 216, 2573–2580 (2013).
Gruys, E., Toussaint, M. J. M., Niewold, T. A. & Koopmans, S. J. Acute phase reaction and acute phase proteins. J. Zhejiang Univ. Sci. B 6, 1045–1056 (2005).
Giansanti, F., Leboffe, L., Pitari, G., Ippoliti, R. & Antonini, G. Physiological roles of ovotransferrin. Biochim. Biophys. Acta BBA – Gen. Subj. 1820, 218–225 (2012).
Sild, E. & Hõrak, P. Nitric Oxide Production: An Easily Measurable Condition Index for Vertebrates. Behav. Ecol. Sociobiol. 63, 959–966 (2009).
Ochsenbein, A. F. & Zinkernagel, R. M. Natural antibodies and complement link innate and acquired immunity. Immunol. Today 21, 624–630 (2000).
Schmid-Hempel, P. & Ebert, D. On the evolutionary ecology of specific immune defence. Trends Ecol. Evol. 18, 27–32 (2003).
Panda, S. & Ding, J. L. Natural Antibodies Bridge Innate and Adaptive Immunity. J. Immunol. 194, 13–20 (2015).
Ochsenbein, A. F. et al. Control of Early Viral and Bacterial Distribution and Disease by Natural Antibodies. Science 286, 2156–2159 (1999).
Reid, R. R., Prodeus, A. P., Khan, W., Hsu, T. & Rosen, F. S. Endotoxin shock in antibody-deficient mice: unraveling the role of natural antibody and complement in the clearance of lipopolysaccharide. 7 (1997).
Belperron, A. A. & Bockenstedt, L. K. Natural Antibody Affects Survival of the Spirochete Borrelia burgdorferi within Feeding Ticks. Infect. Immun. 69, 6456–6462 (2001).
Erni, B., Bonnevie, B. T., Oschadleus, H.-D., Altwegg, R. & Underhill, L. G. Moult: an r package to analyse moult in birds. J. Stat. Softw. 52, 1–23 (2013).
Buehler, D. M., Piersma, T., Matson, K. & Tieleman, B. I. Seasonal Redistribution of Immune Function in a Migrant Shorebird: Annual‐Cycle Effects Override Adjustments to Thermal Regime. Am. Nat. 172, 783–796 (2008).
Gosler, A. G., Greenwood, J. J. D., Baker, J. K. & Davidson, N. C. The field determination of body size and condition in passerines: a report to the British Ringing Committee. Bird Study 45, 92–103 (1998).
R Development Core Team, R. A language and environment for statistical computing. vol. 1 (2006).
Tieleman, B. I., Versteegh, M. A., Klasing, K. C. & Williams, J. B. Constitutive innate immunity of tropical House Wrens varies with season and reproductive activity. The Auk 10 (2019).
Hasselquist, D. & Nilsson, J.-Å. Physiological mechanisms mediating costs of immune responses: what can we learn from studies of birds? Anim. Behav. 83, 1303–1312 (2012).
Dammhahn, M., Dingemanse, N. J., Niemelä, P. T. & Réale, D. Pace-of-life syndromes: a framework for the adaptive integration of behaviour, physiology and life history. Behav. Ecol. Sociobiol. 72, 62 (2018).
Monceau, K. et al. Personality, immune response and reproductive success: an appraisal of the pace-of-life syndrome hypothesis. J. Anim. Ecol. 86, 932–942 (2017).
Horrocks, N. P. C., Matson, K. D., Shobrak, M., Tinbergen, J. M. & Tieleman, B. I. Seasonal patterns in immune indices reflect microbial loads on birds but not microbes in the wider environment. Ecosphere 3, art19 (2012).
Johnson, C. K. et al. Prey choice and habitat use drive sea otter pathogen exposure in a resource-limited coastal system. Proc. Natl. Acad. Sci. USA 106, 2242–2247 (2009).
Cotter, S. C., Simpson, S. J., Raubenheimer, D. & Wilson, K. Macronutrient balance mediates trade-offs between immune function and life history traits. Funct. Ecol. 25, 186–198 (2011).
Martin, L. B., Weil, Z. M. & Nelson, R. J. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Philos. Trans. R. Soc. B Biol. Sci. 363, 321–339 (2008).
Allen, P. C. Nitric oxide production during Eimeria tenella infections in chickens. Poult. Sci. 76, 810–813 (1997).
Read, A. F. & Allen, J. E. The Economics of Immunity. Science 290, 1104–1105 (2000).
Bourgeon, S., Raclot, T., Le Maho, Y., Ricquier, D. & Criscuolo, F. Innate immunity, assessed by plasma NO measurements, is not suppressed during the incubation fast in eiders. Dev. Comp. Immunol. 31, 720–728 (2007).
Bogdan, C., Röllinghoff, M. & Diefenbach, A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 12, 64–76 (2000).
O’Connor, E. A., Hasselquist, D., Nilsson, J.-Å., Westerdahl, H. & Cornwallis, C. K. Wetter climates select for higher immune gene diversity in resident, but not migratory, songbirds. Proc. R. Soc. B Biol. Sci. 287, 20192675 (2020).
Horrocks, N. P. et al. Are antimicrobial defences in bird eggs related to climatic conditions associated with risk of trans-shell microbial infection? Front. Zool. 11, 49 (2014).
Mendes, L., Piersma, T., Hasselquist, D., Matson, K. D. & Ricklefs, R. E. Variation in the innate and acquired arms of the immune system among five shorebird species. J. Exp. Biol. 209, 284–291 (2006).
Nunn, C. L. A Comparative Study of Leukocyte Counts and Disease Risk in Primates. Evolution 56, 177–190 (2002).
Nunn, C. L., Gittleman, J. L. & Antonovics, J. A comparative study of white blood cell counts and disease risk in carnivores. Proc. R. Soc. B Biol. Sci. 270, 347–356 (2003).
Blount, J. D., Houston, D. C., Møller, A. P. & Wright, J. Do individual branches of immune defence correlate? A comparative case study of scavenging and non-scavenging birds. Oikos 102, 340–350 (2003).
Matson, K. D. Are there differences in immune function between continental and insular birds? Proc. R. Soc. B Biol. Sci. 273, 2267–2274 (2006).
Spottiswoode, C. N. Cooperative Breeding and Immunity: A Comparative Study of PHA Response in African Birds. Behav. Ecol. Sociobiol. 62, 963–974 (2008).
Buehler, D. M., Tieleman, B. I. & Piersma, T. Indices of Immune Function are Lower in Red Knots (Calidris canutus) Recovering Protein than in those Storing Fat During Stopover in Delaware Bay. The Auk 127, 394–401 (2010).
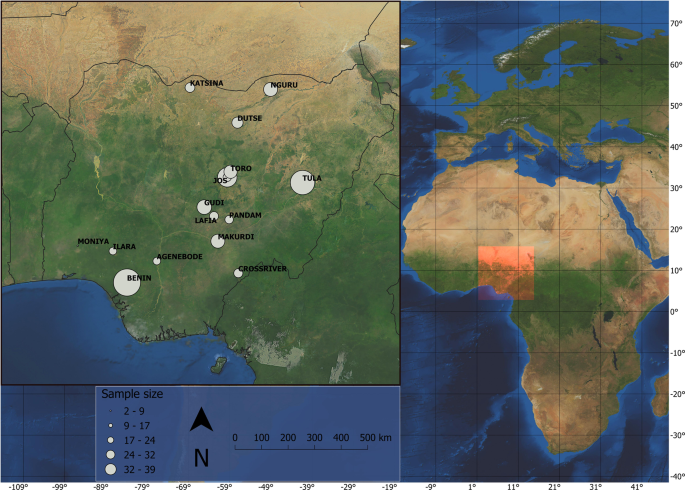
Nwaogu, C. J. Avian life in a seasonally arid tropical environment: adaptations and mechanisms in breeding, moult and immune function. Dr. Thesis Univ. Gron. 237 (2019).
Source: Ecology - nature.com


