Wright, I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827 (2004).
Chave, J. et al. Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366 (2009).
Westoby, M., Jurado, E. & Leishman, M. R. Comparative evolutionary ecology of seed size. Trends Ecol. Evol. 7, 368–372 (1992).
Iversen, C. M. et al. The unseen iceberg: plant roots in arctic tundra. N. Phytologist 205, 34–58 (2015).
Westoby, M. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 199, 213–227 (1998).
Westoby, M. & Wright, I. J. Land-plant ecology on the basis of functional traits. Trends Ecol. Evol. 21, 261–268 (2006).
Reich, P. B. The world-wide ‘fast-slow’ plant economics spectrum: a traits manifesto. J. Ecol. 102, 275–301 (2014).
Díaz, S. et al. The global spectrum of plant form and function. Nature 529, 167–171 (2016).
McGill, B. J., Enquist, B. J., Weiher, E. & Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185 (2006).
Cornwell, W. K. & Ackerly, D. D. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecol. Monogr. 79, 109–126 (2009).
Suding, K. N. et al. Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Glob. Change Biol. 14, 1125–1140 (2008).
Lavorel, S. & Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: revisting the Holy Grail. Funct. Ecol. 16, 545–556 (2002).
Bjorkman, A. D. et al. Plant functional trait change across a warming tundra biome. Nature 562, 57–62 (2018).
Moran, E. V., Hartig, F. & Bell, D. M. Intraspecific trait variation across scales: Implications for understanding global change responses. Glob. Change Biol. 22, 137–150 (2016).
Kattge, J. et al. TRY-a global database of plant traits. Glob. Change Biol. 17, 2905–2935 (2011).
Reich, P. B., Walters, M. B. & Ellsworth, D. S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl Acad. Sci. USA 94, 13730–13734 (1997).
Freschet, G. T., Cornelissen, J. H. C., van Logtestijn, R. S. P. & Aerts, R. Evidence of the ‘plant economics spectrum’ in a subarctic flora. J. Ecol. 98, 362–373 (2010).
Myers-Smith, I. H., Thomas, H. J. D. & Bjorkman, A. D. Plant traits inform predictions of tundra responses to global change. N. Phytologist 221, 1742–1748 (2019).
Wigley, B. J. et al. Leaf traits of African woody savanna species across climate and soil fertility gradients: evidence for conservative versus acquisitive resource-use strategies. J. Ecol. 104, 1357–1369 (2016).
Shipley, B. et al. Reinforcing loose foundation stones in trait-based plant ecology. Oecologia 1–9 (2016). https://doi.org/10.1007/s00442-016-3549-x.
Siefert, A. et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol. Lett. 18, 1406–1419 (2015).
Anderegg, L. D. L. et al. Within-species patterns challenge our understanding of the leaf economics spectrum. Ecol. Lett. 21, 734–744 (2018).
Laughlin, D. C. et al. Intraspecific trait variation can weaken interspecific trait correlations when assessing the whole-plant economic spectrum. Ecol. Evol. 7, 8936–8949 (2017).
De La Riva, E. G., Olmo, M., Poorter, H., Ubera, J. L. & Villar, R. Leaf mass per area (LMA) and its relationship with leaf structure and anatomy in 34 mediterranean woody species along a water availability gradient. PLoS ONE 11, e0148788 (2016).
Fajardo, A. & Piper, F. I. Intraspecific trait variation and covariation in a widespread tree species (Nothofagus pumilio) in southern Chile. N. Phytologist 189, 259–271 (2011).
Messier, J., McGill, B. J., Enquist, B. J. & Lechowicz, M. J. Trait variation and integration across scales: is the leaf economic spectrum present at local scales? Ecography 40, 685–697 (2017).
Albert, C. H., Grassein, F., Schurr, F. M., Vieilledent, G. & Violle, C. When and how should intraspecific variability be considered in trait-based plant ecology? Perspect. Plant Ecol. Evol. Syst. 13, 217–225 (2011).
Jetz, W. et al. Monitoring plant functional diversity from space. Nat. Plants 2, 1–5 (2016).
Pearson, R. G. et al. Shifts in Arctic vegetation and associated feedbacks under climate change. Nat. Clim. Change 3, 673–677 (2013).
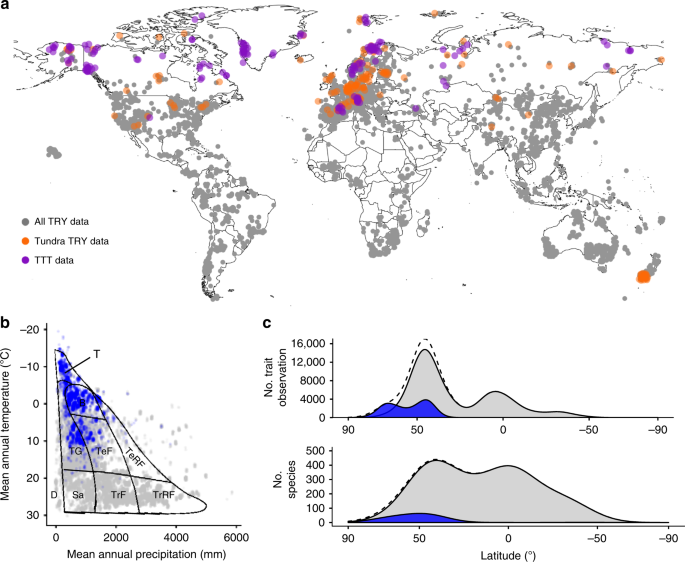
Bjorkman, A. D. et al. Tundra Trait Team: a database of plant traits spanning the tundra biome. Glob. Ecol. Biogeogr. 27, 1402–1411 (2018).
Manning, P. et al. Simple measures of climate, soil properties and plant traits predict national-scale grassland soil carbon stocks. J. Appl. Ecol. 52, 1188–1196 (2015).
Billings, W. D. Constraints to plant growth, reproduction, and establishment in arctic environments. Arct. Alp. Res. 19, 357 (1987).
Thomas, H. J. D. et al. Traditional plant functional groups explain variation in economic but not size-related traits across the tundra biome. Glob. Ecol. Biogeogr. 28, 78–95 (2019).
Kunstler, G. et al. Plant functional traits have globally consistent effects on competition. Nature 529, 1–15 (2016).
Bjorkman, A. D., Vellend, M., Frei, E. R. & Henry, G. H. R. Climate adaptation is not enough: warming does not facilitate success of southern tundra plant populations in the high Arctic. Glob. Change Biol. 23, 1540–1551 (2017).
Pérez-Ramos, I. M., Matías, L., Gómez-Aparicio, L. & Godoy, Ó. Functional traits and phenotypic plasticity modulate species coexistence across contrasting climatic conditions. Nat. Commun. 10, 1–11 (2019).
Elmendorf, S. C. et al. Experiment, monitoring, and gradient methods used to infer climate change effects on plant communities yield consistent patterns. Proc. Natl Acad. Sci. USA 112, 448–452 (2015).
Hudson, J. M. G., Henry, G. H. R. & Cornwell, W. K. Taller and larger: shifts in Arctic tundra leaf traits after 16 years of experimental warming. Glob. Change Biol. 17, 1013–1021 (2011).
Myers-Smith, I. H. et al. Eighteen years of ecological monitoring reveals multiple lines of evidence for tundra vegetation change. Ecol. Monogr. 89, e01351 (2019).
Hoffmann, A. A. & Merilä, J. Heritable variation and evolution under favourable and unfavourable conditions. Trends Ecol. Evol. 14, 96–101 (1999).
Baruah, G., Molau, U., Bai, Y. & Alatalo, J. M. Community and species-specific responses of plant traits to 23 years of experimental warming across subarctic tundra plant communities. Sci. Rep. 7, 2571 (2017).
Violle, C. et al. The return of the variance: Intraspecific variability in community ecology. Trends Ecol. Evol. 27, 244–252 (2012).
Opedal, Ø. H., Armbruster, W. S. & Graae, B. J. Linking small-scale topography with microclimate, plant species diversity and intra-specific trait variation in an alpine landscape. Plant Ecol. Diversity 8, 305–315 (2015).
Elberling, B. Annual soil CO2 effluxes in the High Arctic: The role of snow thickness and vegetation type. Soil Biol. Biochem. 39, 646–654 (2007).
McGraw, J. B. Experimental ecology of Dryas octopetala ecotypes. III. Environ. Factors Plant Growth Arct. Alp. Res. 17, 229–239 (1985).
Soudzilovskaia, N. A. et al. Functional traits predict relationship between plant abundance dynamic and long-term climate warming. Proc. Natl Acad. Sci. USA 110, 18180–18184 (2013).
Wullschleger, S. D. et al. Plant functional types in Earth system models: past experiences and future directions for application of dynamic vegetation models in high-latitude ecosystems. Ann. Bot. 114, 1–16 (2014).
Myers-Smith, I. H. et al. Climate sensitivity of shrub growth across the tundra biome. Nat. Clim. Change 5, 887–891 (2015).
Elmendorf, S. C. et al. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat. Clim. Change 2, 453–457 (2012).
Bliss, L. C., Heal, O. W. & Moore, J. J. Tundra Ecosystems: A Comparative Analysis. (CUP Archive, 1981).
Henry, G. H. R. & Molau, U. Tundra plants and climate change: the International Tundra Experiment (ITEX). Glob. Change Biol. 3, 1–9 (1997).
Steinbauer, M. J. et al. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 556, 231–234 (2018).
Weiher, E. et al. Challenging theophrastus: a common core list of plant traits for functional ecology. J. Vegetation Sci. 10, 609–620 (1999).
Choat, B. et al. Global convergence in the vulnerability of forests to drought. Nature 491, 752–755 (2012).
Burrascano, S. et al. Wild boar rooting intensity determines shifts in understorey composition and functional traits. Community Ecol. 16, 244–253 (2015).
Byun, C., de Blois, S. & Brisson, J. Plant functional group identity and diversity determine biotic resistance to invasion by an exotic grass. J. Ecol. 101, 128–139 (2013).
Campbell, C. et al. Acclimation of photosynthesis and respiration is asynchronous in response to changes in temperature regardless of plant functional group. N. Phytologist 176, 375–389 (2007).
Castro-Díez, P., Puyravaud, J. P., Cornelissen, J. H. C. & Villar-Salvador, P. Stem anatomy and relative growth rate in seedlings of a wide range of woody plant species and types. Oecologia 116, 57–66 (1998).
Cerabolini, B. E. L. et al. Can CSR classification be generally applied outside Britain? Plant Ecol. 210, 253–261 (2010).
Ciocarlan, V. The illustrated Flora of Romania. Pteridophyta et Spermatopyta. (Editura Ceres, 2009).
Cornelissen, J. H. C. An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. J. Ecol. 84, 573 (1996).
Cornelissen, J. H. C. et al. Functional traits of woody plants: correspondence of species rankings between field adults and laboratory-grown seedlings? J. Vegetation Sci. 14, 311–322 (2003).
Cornelissen, J. H. C. et al. Leaf digestibility and litter decomposability are related in a wide range of subarctic plant species and types. Funct. Ecol. 18, 779–786 (2004).
Cornelissen, J. H. C., Diez, P. C. & Hunt, R. Seedling growth, allocation and leaf attributes in a wide range of woody plant species and types. J. Ecol. 84, 755 (1996).
Cornwell, W. K. et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 11, 1065–1071 (2008).
Craine, J. M. et al. Functional consequences of climate change-induced plant species loss in a tallgrass prairie. Oecologia 165, 1109–1117 (2011).
Craine, J. M., Towne, E. G., Ocheltree, T. W. & Nippert, J. B. Community traitscape of foliar nitrogen isotopes reveals N availability patterns in a tallgrass prairie. Plant Soil 356, 395–403 (2012).
Craine, J. M. et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. N. Phytologist 183, 980–992 (2009).
Craine, J. M., Lee, W. G., Bond, W. J., Williams, R. J. & Johnson, L. C. Environmental constraints on a global relationship among leaf and root traits of grasses. Ecology 86, 12–19 (2005).
Diaz, S. et al. The plant traits that drive ecosystems: evidence from three continents. J. Vegetation Sci. 15, 295–304 (2004).
Dainese, M. & Bragazza, L. Plant traits across different habitats of the Italian Alps: a comparative analysis between native and alien species. Alp. Bot. 122, 11–21 (2012).
Everwand, G., Fry, E. L., Eggers, T. & Manning, P. Seasonal variation in the relationship between plant traits and grassland carbon and water fluxes. J. Ecol. 17, 1095–1108 (2014).
Fitter, A. H. & Peat, H. J. The ecological flora database. J. Ecol. 82, 415–425 (1994).
Atkin, O. K., Westbeek, M. H. M., Cambridge, M. L., Lambers, H. & Pons, H. Leaf Respiration in Light and Darkness. Plant Physiol. 113, 961–965 (1997).
Fonseca, C. R., Overton, J. M., Collins, B. & Westoby, M. Shifts in trait-combinations along rainfall and phosphorus gradients. J. Ecol. 88, 964–977 (2000).
Fry, E. L., Power, S. A. & Manning, P. Trait-based classification and manipulation of plant functional groups for biodiversity-ecosystem function experiments. J. Vegetation Sci. 25, 248–261 (2014).
Gallagher, R. V. & Leishman, M. R. A global analysis of trait variation and evolution in climbing plants. J. Biogeogr. 39, 1757–1771 (2012).
Garnier, E. et al. Assessing the effects of land-use change on plant traits, communities and ecosystem functioning in grasslands: A standardized methodology and lessons from an application to 11 European sites. Ann. Bot. 99, 967–985 (2007).
Green, W. USDA PLANTS Compilation, version 1, 09-02-02. NRCS: The PLANTS Database (2009).
Han, W., Fang, J., Guo, D. & Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. N. Phytologist 168, 377–385 (2005).
Guy, A. L., Mischkolz, J. M. & Lamb, E. G. Limited effects of simulated acidic deposition on seedling survivorship and root morphology of endemic plant taxa of the Athabasca Sand Dunes in well-watered greenhouse trials. Botany 91, 176–181 (2013).
Hickler, T. Plant functional types and community characteristics along environmental gradients on Oland’s Great Alvar (Sweden). (University of Lund, Sweden, 1999).
Fagúndez, J. & Izco, J. Seed morphology of two distinct european species of Erica L. (Ericaceae). Acta Botanica Malacit. 33, 1–9 (2008).
Kühn, I., Durka, W. & Klotz, S. BiolFlor-A new plant-trait database as a tool for plant invasion ecology. Diversity Distrib. 10, 363–365 (2004).
Bahn, M. et al. Leaf photosynthesis, nitrogen contents and specific leaf area of grassland species in mountain ecosystems under different land use. in Land use changes in European mountain ecosystems: ECOMONT concepts and results. Blackwell, Vienna, Austria 247–255 (1999).
Kattge, J., Knorr, W., Raddatz, T. & Wirth, C. Quantifying photosynthetic capacity and its relationship to leaf nitrogen content for global-scale terrestrial biosphere models. Glob. Change Biol. 15, 976–991 (2009).
Kazakou, E., Vile, D., Shipley, B., Gallet, C. & Garnier, E. Co-variations in litter decomposition, leaf traits and plant growth in species from a Mediterranean old-field succession. Funct. Ecol. 20, 21–30 (2006).
Kerkoff, A., Fagan, W. F., James, J., Elser & Brian, J. Enquist. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. Am. Naturalist 168, E103–E122 (2006).
Kichenin, E., Wardle, D. A., Peltzer, D. A., Morse, C. W. & Freschet, G. T. Contrasting effects of plant inter- and intraspecific variation on community-level trait measures along an environmental gradient. Funct. Ecol. 27, 1254–1261 (2013).
Kleyer, M. et al. The LEDA Traitbase: a database of life-history traits of the Northwest European flora. J. Ecol. 96, 1266–1274 (2008).
Louault, F., Pillar, V. D., Aufrère, J., Garnier, E. & Soussana, J. F. Plant traits and functional types in response to reduced disturbance in a semi-natural grassland. J. Vegetation Sci. 16, 151–160 (2005).
Loveys, B. R. et al. Thermal acclimation of leaf and root respiration: an investigation comparing inherently fast- and slow-growing plant species. Glob. Change Biol. 9, 895–910 (2003).
Moretti, M. & Legg, C. Combining plant and animal traits to assess community functional responses to disturbance. Ecography 32, 299–309 (2009).
Medlyn, B. E. et al. Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. N. Phytologist 149, 247–264 (2001).
Mencuccini, M. The ecological significance of long-distance water transport: Short-term regulation, long-term acclimation and the hydraulic costs of stature across plant life forms. Plant Cell Environ. 26, 163–182 (2003).
Bakker, C., Rodenburg, J. & Van Bodegom, P. M. Effects of Ca- and Fe-rich seepage on P availability and plant performance in calcareous dune soils. in. Plant Soil 275, 111–122 (2005).
Meziane, D. & Shipley, B. Interacting determinants of specific leaf area in 22 herbaceous species: effects of irradiance and nutrient availability. Plant Cell Environ. 22, 447–459 (1999).
Milla, R. & Reich, P. B. Multi-trait interactions, not phylogeny, fine-tune leaf size reduction with increasing altitude. Ann. Bot. 107, 455–465 (2011).
Niinemets, U. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 82, 453–469 (2001).
Ogaya, R. & Peñuelas, J. Comparative field study of Quercus ilex and Phillyrea latifolia: photosynthetic response to experimental drought conditions. Environ. Exp. Bot. 50, 137–148 (2003).
Onoda, Y. et al. Global patterns of leaf mechanical properties. Ecol. Lett. 14, 301–312 (2011).
Ordoñez, J. C. et al. Plant strategies in relation to resource supply in mesic to wet environments: does theory mirror nature? Am. Naturalist 175, 225–239 (2010).
Paula, S. et al. Fire-related traits for plant species of the Mediterranean Basin. Ecology 90, 46470 (2009).
Peco, B., De Pablos, I., Traba, J. & Levassor, C. The effect of grazing abandonment on species composition and functional traits: the case of dehesa grasslands. Basic Appl. Ecol. 6, 175–183 (2005).
Adler, P. B., Milchunas, D. G., Lauenroth, W. K., Sala, O. E. & Burke, I. C. Functional traits of graminoids in semi-arid steppes: A test of grazing histories. J. Appl. Ecol. 41, 653–663 (2004).
Pierce, S., Brusa, G., Sartori, M. & Cerabolini, B. E. L. Combined use of leaf size and economics traits allows direct comparison of hydrophyte and terrestrial herbaceous adaptive strategies. Ann. Bot. 109, 1047–1053 (2012).
Bakker, C., Van Bodegom, P. M., Nelissen, H. J. M., Ernst, W. H. O. & Aerts, R. Plant responses to rising water tables and nutrient management in calcareous dune slacks. Plant Ecol. 185, 19–28 (2006).
Pierce, S., Brusa, G., Vagge, I. & Cerabolini, B. E. L. Allocating CSR plant functional types: the use of leaf economics and size traits to classify woody and herbaceous vascular plants. Funct. Ecol. 27, 1002–1010 (2013).
Pierce, S., Ceriani, R. M., DE Andreis, R., Luzzaro, A. & Cerabolini, B. The leaf economics spectrum of Poaceae reflects variation in survival strategies. Plant Biosyst. 141, 337–343 (2007).
Pierce, S., Luzzaro, A., Caccianiga, M., Ceriani, R. M. & Cerabolini, B. Disturbance is the principal α-scale filter determining niche differentiation, coexistence and biodiversity in an alpine community. J. Ecol. 95, 698–706 (2007).
Poorter, H., Niinemets, Ü., Poorter, L., Wright, I. J. & Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. N. Phytologist 182, 565–588 (2009).
Poschlod, P., Kleyer, M., Jackel, A.-K., Dannemann, A. & Tackenberg, O. BIOPOP—A database of plant traits and internet application for nature conservation. Folia Geobotanica 38, 263–271 (2003).
Prentice, I. C. et al. Evidence of a universal scaling relationship for leaf CO2 drawdown along an aridity gradient. N. Phytologist 190, 169–180 (2011).
Preston, K. A., Cornwell, W. K. & DeNoyer, J. L. Wood density and vessel traits as distinct correlates of ecological strategy in 51 California coast range angiosperms. N. Phytologist 170, 807–818 (2006).
Price, C. A. & Enquist, B. J. Scaling mass and morphology in leaves: an extension of the wbe model. Ecology 88, 1132–1141 (2007).
Pyankov, V. I., Kondratchuk, A. V. & Shipley, B. Leaf structure and specific leaf mass: the alpine desert plants of the Eastern Pamirs, Tadjikistan. N. Phytologist 143, 131–142 (1999).
Quested, H. M. et al. Decomposition of sub-arctic plants with differing nitrogen economies: a functional role for hemiparasites. Ecology 84, 3209–3221 (2003).
Blonder, B. et al. The leaf-area shrinkage effect can bias paleoclimate and ecology research. Am. J. Bot. 99, 1756–1763 (2012).
Reich, P. B., Oleksyn, J. & Wright, I. J. Leaf phosphorus influences the photosynthesis-nitrogen relation: a cross-biome analysis of 314 species. Oecologia 160, 207–212 (2009).
Reich, P. B. et al. Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol. Lett. 11, 793–801 (2008).
Kew, R. B. G. Seed information database (SID). Version 7.1. http://www.kew.org/data/sid(2008).
Scherer-Lorenzen, M., Schulze, E. D., Don, A., Schumacher, J. & Weller, E. Exploring the functional significance of forest diversity: A new long-term experiment with temperate tree species (BIOTREE). Perspect. Plant Ecol. Evol. Syst. 9, 53–70 (2007).
Schweingruber, F. & Landolt, W. The xylem database. Swiss Federal Research Institute WSL http://www.wsl.ch/dendropro/xylemdb/ (2005).
Sheremet’ev, S. N. Herbs on the soil moisture gradient (water relations and the structural-functional organization). (KMK, Moscow, 2005).
Shipley, B. Trade-offs between net assimilation rate and specific leaf area in determining relative growth rate: relationship with daily irradiance. Funct. Ecol. 16, 682–689 (2002).
Shipley, B. Structured interspecific determinants of SLA in 34 species of herbaceous angiosperms. Funct. Ecol. 9, 312–319 (1995).
Shipley, B. & Lechowicz, M. J. The functional co-ordination of leaf morphology, nitrogen concentration, and gas exchange in 40 wetland species. Ecoscience 7, 183–194 (2000).
Shipley, B. & Parent, M. Germination responses of 64 wetland species in relation to seed size, minimum Time to reproduction and seedling relative growth rate. Source Funct. Ecol. 5, 111–118 (1991).
Blonder, B., Violle, C. & Enquist, B. J. Assessing the causes and scales of the leaf economics spectrum using venation networks in Populus tremuloides. J. Ecol. 101, 981–989 (2013).
Shipley, B. & Vu, T. T. Dry matter content as a measure of dry matter concentration in plants and their parts. N. Phytologist 153, 359–364 (2002).
Spasojevic, M. J. & Suding, K. N. Inferring community assembly mechanisms from functional diversity patterns: The importance of multiple assembly processes. J. Ecol. 100, 652–661 (2012).
Tucker, S. S., Craine, J. M. & Nippert, J. B. Physiological drought tolerance and the structuring of tallgrass prairie assemblages. Ecosphere 2, 48 (2011).
Van Bodegom, P. M., Sorrell, B. K., Oosthoek, A., Bakker, C. & Aerts, R. Separating the effects of partial submergence and soil oxygen demand on plant physiology. Ecology 89, 193–204 (2008).
Minden, V. & Kleyer, M. Testing the effect-response framework: key response and effect traits determining above-ground biomass of salt marshes. J. Vegetation Sci. 22, 387–401 (2011).
Minden, V., Andratschke, S., Spalke, J., Timmermann, H. & Kleyer, M. Plant trait-environment relationships in salt marshes: Deviations from predictions by ecological concepts. Perspect. Plant Ecol. Evolution Syst. 14, 183–192 (2012).
Vergutz, L., Manzoni, S., Porporato, A., Novais, R. F. & Jackson, R. B. A Global Database of Carbon and Nutrient Concentrations of Green and Senesced Leaves. (2012).
Vile, D. Significations fonctionnelle et ecologique des traits des especes vegetales: exemple dans une succession post-cultural mediterraneenne et generalisations. (2005).
Han, W. et al. Floral, climatic and soil pH controls on leaf ash content in China’s terrestrial plants. Glob. Ecol. Biogeogr. 21, 376–382 (2012).
Wirth, C. & Lichstein, J. The imprint of species turnover on old-growth forest carbon balances-Insights from a trait-based model of forest dynamics. Old Growth Forest. SE-5 207, 81–113 (2009).
Sandel, B., Corbin, J. D. & Krupa, M. Using plant functional traits to guide restoration: a case study in California coastal grassland. Ecosphere 2, 1–16 (2011).
Chen, Y., Han, W., Tang, L., Tang, Z. & Fang, J. Leaf nitrogen and phosphorus concentrations of woody plants differ in responses to climate, soil and plant growth form. Ecography 36, 178–184 (2013).
Medlyn, B. E. et al. Effects of elevated CO2 on photosynthesis in European forest species: a meta-analysis of model parameters. Plant Cell Environ. 22, 1475–1495 (1999).
Cayuela, L., Granzow-de la Cerda, Í., Albuquerque, F. S. & Golicher, D. J. Taxonstand: an R package for species names standardisation in vegetation databases. Methods Ecol. Evol. 3, 1078–1083 (2012).
McIlroy, D., Brownrigg, R., Minka, T. P. & Bivand, R. mapproj: Map Projections. 1–2 (2014).
Büntgen, U., Psomas, A. & Schweingruber, F. H. Introducing wood anatomical and dendrochronological aspects of herbaceous plants: Applications of the Xylem Database to vegetation science. J. Vegetation Sci. 25, 967–977 (2014).
Chapin, F. S. III, Matson, P. A. & Vitousek, P. Principles of terrestrial ecosystem ecology. Springe. Sci. Bus. Media. https://doi.org/10.5860/choice.40-2771 (2011).
Source: Ecology - nature.com



