Osmia bicornis population management
Osmia bicornis cocoons reared at the arboretum of the Botanical Garden of the University of Rostock (Germany) were obtained from Johann-Christoph Kornmilch (bienenhotel.de, Rostock, Germany). The cocoons were shipped in January and stored in the dark at 4°–7 °C. To induce adult emergence, cocoons were placed in cages (30 × 30 × 30 cm, Aerarium, bioform, Nürnberg, Germany) under natural light at room temperature (1st of March–4th of May 2016 and 2018; average temperature 22.6 °C, min 20.0 °C, max. 24.2 °C; relative humidity 62%, min. 58.8%, max. 72%, datalogger EL-USB-2, Lascar electronics, Whiteparish). We separated female and male cocoons based on size, and kept them in separate cages for the whole study. Approximately 24 hours after emergence, we transferred the bees to new cages in groups of 5 to 10 individuals, where the exposure to thiacloprid took place. We conducted three independent replicates (cages) per treatment and sex for each immune assay.
Test solutions
A stock solution of 2 mg/ml thiacloprid (Sigma Aldrich, St. Louis, USA; purity 99.9%) in acetone was prepared in a glass flask and stored in the dark at 15 °C until use. The stock solution was added to a 50% feeding solution of invert sugar (Ambrosia, Germany) in distilled water (w/v) to reach three test concentrations: 100, 200, and 555 µg/kg (henceforward referred to as treatment 100, 200 and 555). The final concentration of acetone in the test solutions was adjusted to 0.0086% (v:v) in all treatments.
A pollen patty was prepared from pollen collected by honey bees at the Bee Institute Kirchhain or obtained from Imkereibedarf Bährle (Aschaffenburg, Germany). The pollen was stored at −20 °C for 8–10 months and then pulverized in a coffee grinder. The resulting pollen powder was mixed with the thiacloprid-spiked feeding solution to obtain the same concentrations as the feeding solution (100, 200, 555 µg of thiacloprid per kg of pollen patty).
Neonicotinoid exposure
Test cages were placed close to each other (~2 cm), so bees of both sexes could see, hear and smell each other. Fragments of egg carton and 5 cm segments of black plastic drinking straws were placed in each flight cage as hiding sites. Cages were kept at room temperature under natural light conditions.
Caps of 1.5 ml microcentrifuge tubes (Eppendorf, Hamburg, Germany) were cut off and used as containers for the feeding solution (two caps per cage) and pollen paste (two caps per cage). The bottoms of the caps were coloured red or blue and placed on a green piece of cardboard to attract the bees. Both females and males found the food sources immediately, and feeding was observed frequently.
Each cage was assigned to one of four thiacloprid treatments and exposed for three days. Feeding caps were weighed and renewed every day. For each treatment group and sex, three cages of 6 to 10 individuals were set up on independent dates. In 2016, we tested the 0, 100 and 200 µg/kg treatments (Table 2). Then, based on the discrepancies observed between the dosage per body weight in males and females, additional experiments with 0 and 555 treatments were conducted (three groups for each treatment and sex on independent dates in 2018) to obtain data on a dosage taken up by females equivalent to the 200 treatment in males (Table 2). In order to minimize the variance in the following functional immune assays caused by different experimenters, only one person at a time analysed the three treatment groups (Suppl. Tables 1 and 2).
Hemolymph extraction and total hemocyte counts
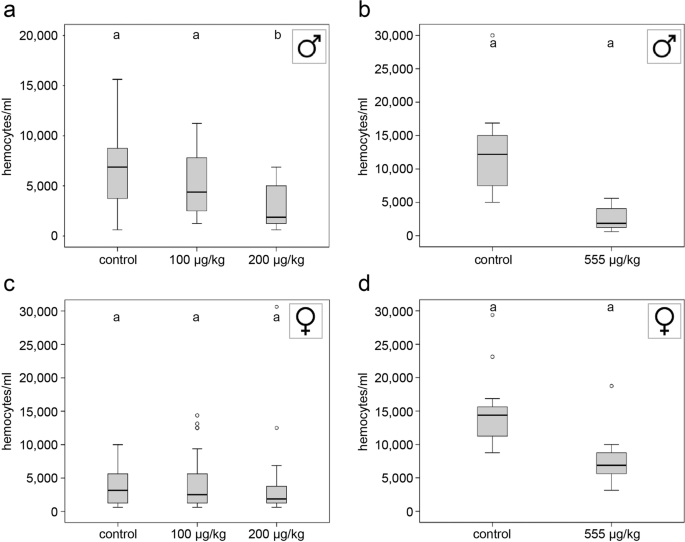
Bees were anesthetized on ice, and hemolymph was extracted by inserting a microinjection needle (Hartenstein, Würzburg, Germany) into the proximal abdomen between the 3rd and 4th tergum25,26. Hemolymph (1 µl) was transferred to PCR-tubes (Biozym, Hessisch Oldendorf, Germany) containing 1 µl of DAPI-staining solution (4’,6-diamidino-2-phenylindole; 1:100 dilution, lifetechnologies, Carlsbad, California, USA) and 3 µl PBS (pH 7.4; Sigma Aldrich, St. Louis, USA). Hemocytes were counted in a counting chamber (Bürker, Carl Roth, Karlsruhe, Germany) under a phase contrast/fluorescent microscope (Leica DMIL, Leica camera DFC 420 C)25,26.
Encapsulation response
A 2.5 mm nylon filament was partly inserted into the abdomen of anesthetized bees as previously described for honeybees25,26. After implantation, females were transferred to 1.5 ml microcentrifuge tubes and males to PCR-tubes with holes poked through the cap and sidewalls. After four hours at room temperature, the nylon filament was extracted, fixed in formaldehyde solution for at least 1 hour, rinsed three times in PBS, and subsequently mounted in glycerol (85%, Carl Roth). For each explant, three pictures were taken at different focal depths. The mean grey value per filament was calculated using image analysis software and taken as a measure of melanisation59. The mean grey value of a non-implanted filament was subtracted from the mean grey value of the implanted filaments25.
Antimicrobial response
On day two of the exposure phase, the immune system was challenged by the injection of 1 µl of heat-inactivated Escherichia coli (OD 0.5)25,26. Hemolymph was collected and stored at −20 °C. Bacterial test plates (ø 9 cm) were prepared by adding 0.8 ml of live Micrococcus luteus bacteria suspension (OD 0.5) to 150 ml of sterile broth medium (48 °C, 1.5 g Agar No. 1, Oxoid; 3.75 g nutrient broth, Applichem). For each test plate, five holes (ø 3.33 mm) were punched into the agar with a 1 ml pipette tip, and 1 µl of hemolymph solution was added to each hole. Plates were incubated at 38 °C overnight, and the diameter of inhibition zones was measured with a digital calliper25,26.
Repeatability of measurements
To investigate possible sources of variance and estimate the technical repeatability of our functional measurements of hemocyte density, antimicrobial activity of the hemolymph, and melanisation response, we conducted additional experiments. For these experiments, we collected adult Apis mellifera workers from an apparently healthy colony that was not treated against Varroa destructor (December 18, 2019). For hemocyte counts, we extracted hemolymph as described before26. Two experimenters (for details, see Suppl. Table 1) counted the hemocytes of 30 bees independently. Since hemocytes are mobile, we counted the cells as fast as possible.
For the melanisation response, we implanted and prepared 30 transparent nylon fibres as described before26. A single experimenter (Suppl. Table 1) made the microscopic pictures of the same implants twice and conducted the subsequent image analysis independently from each other. To reduce the technical variance, we led the microscope lamp warm up for 30 min before we started to take the pictures.
To estimate for the methodological repeatability of inhibition zone assays, we applied 1 µl of a standard solution of lysozyme (1 mg/1 ml, Applichem, Darmstadt, Germany) on M. flavus inoculated petri dishes. To cover different technical sources of variance, we tested three scenarios (a) pipetting done by two persons, (b) measuring of the inhibition zone diameters done by two persons, (c) repeated measurements of the inhibition zone diameters done by a single person.
Statistical methods
All statistical tests were run with SPSS for Windows (v. 20). Total hemocyte counts, melanisation/mean grey values, and mean diameters of inhibition zones were not normally distributed. Thus, non-parametric statistics were applied25,26. Each immunocompetence measure was compared across treatments using Kruskal–Wallis tests (KWT) followed by post-hoc pair-wise comparisons with Mann–Whitney U tests (MWU) and Bonferroni corrections (when more than two groups were compared)25,26. In order to calculate the repeatability of the functional measurements, we first applied a one-way ANOVA and subsequently calculated the approximate repeatability values from the F ratio and mean squares among groups/mean squares within groups according to Lessells and Boag60.
Ethics
Ethical approval and the licences were obtained from the Hessian Regional Council of Giessen (RPGI), Germany.
Source: Ecology - nature.com


