We proposed that domestic cats, with a higher than average intake of fish-based foods compared to domestic dogs and Scottish wildcats, have a higher intake of dietary arsenic that bioaccumulates in their kidneys, leading to interstitial nephritis that underpins CKD. Our data only partially support this contention. In our sampled population of domestic cats and dogs submitted to the University of Nottingham veterinary pathology service, we found a high incidence of renal lesions in domestic cats, whom had increased levels of arsenic in their kidney tissue and urine, relative to domestic dogs and Scottish wildcats. However, total arsenic content in kidney and urine were very low per se. Whilst, animals with CIN also had higher urine arsenic, any differences were again biologically small. Furthermore, we determined the form of arsenic present in companion animal foods and thus likely to bioaccumulate in their tissues, to be almost entirely arsenobetaine – an organic, non-toxic arsenic species. Nevertheless, we show for the first time that a feral felid, the Scottish wildcat, had barely detectable renal lesions, despite the size of their kidneys indicating a fully-grown adult animal. In contrast, it is relatively rare for adult domestic cats to be free from any renal lesion. Thus, our data compliments other work to suggest that domestic environment appears a risk-factor per se for cats to develop CKD. Whilst diet and genetics may partly underpin this risk, we exclude the possibility that increased intake of organic arsenic, as is often found at high levels in fish-based diets, is a likely causative factor.
Renal histopathology and prevalence of chronic interstitial nephritis in felids
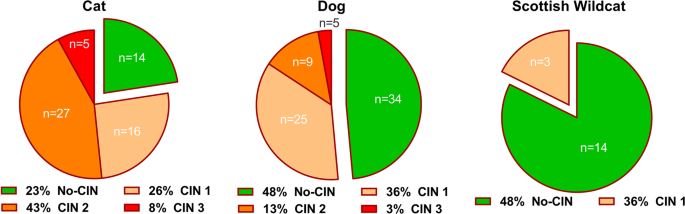
In our population of domestic cats and dogs from a regional pathology unit over an 1.5-2 year collection period we found, unsurprisingly, that more kidneys from domestic cats, than those from dogs and Scottish wildcats, had CIN. A high proportion of those cats had moderate-severe CIN. This corroborates prior work indicating that CKD, for which CIN is the most common renal lesion4,34, is frequently observed in domestic cats, but not in dogs1. Similar to the current study, Chakrabarti and colleagues observed a correlation between scores for renal inflammation and fibrosis4. However, McLeland et al. reported that scores for inflammation were generally greater than scores for fibrosis at each stage of CKD5. This suggests, as is commonly thought, that inflammation generally precedes fibrosis in any organ35. In the current study, analysing mineral content of kidney tissue and urine from cats and dogs, grouped by the presence or absence of mononuclear cell inflammation (CIN), as opposed to fibrosis score, will have allowed identification of a greater number of potential cases of CKD, including those at an earlier stage of progression to later CKD. Presence of inflammation in the renal interstitium has been associated with azotaemia in cats4,5. Thus, while clinical parameters, such as serum creatinine, used to assess the severity of azotaemia and thus stage CKD according to the IRIS system, were not measured in the current study, it is likely that our CIN scores 2 and 3 are indicative of CKD. However, direct correlation between the severities of interstitial inflammation and azotaemia is not a consistent finding4,5. Therefore, it should be noted that the CIN scores used in the current study indicate differing degrees of CIN and not necessarily the differing severity of CKD.
It has also been noted that when captive or truly-wild cat sub-species such as cheetahs, leopards, lynx, cougars, jaguars, ocelots, lions and tigers have been necropsied, minimal inflammatory aggregates and fibrosis are occasionally observed, similar to the data presented here. The Scottish wildcat (Felis silvestris grampia) is one of three main sub-species of wildcat (Felis silvestris)36. However, most domestic cats are believed to descend from another wildcat sub-species, the African wildcat (Felis silvestris lybica)37. Wildcats have not inhabited England, Wales or southern Scotland since the mid-19th century; in fact, the Scottish wildcat is endemic to northern Scotland38. Despite probable introgression with domestic cats, creating a population of wild-living cats possessing both domestic- and wild-cat genes39, the Scottish wildcat can be distinguished from the domestic cat (Felis silvestris catus) and the European wildcat (Felis silvestris silvestris) based on coat colour, tail shape and markings, skull measurements and gut length36. The Scottish wildcat is truly wild and has been a protected species under Schedule 5 of the Wildlife and Countryside Act since 198839. Thus, our finding of higher prevalence of CKD in domestic cats than in the Scottish wildcat, may be attributable to aspects of domestication such as diet fed. Nevertheless, we cannot exclude either a subtle genetic contribution, since Scottish wildcats have been proposed as a distinct species, or other environmental factors that may contribute to differences in disease prevalence between the two populations of felids. For example, stress has been proposed to cause renal disease in captive, compared to free-ranging, wild cats40. Renal disease is an age-related disease in all felids and other species studied to date40,41,42,43. It would be reasonable to conclude that the average age of the Scottish Wildcats in our sample, despite being adult, was less than the average age of our domestic cats, limiting the opportunity for renal lesions to develop. Likewise, in our study, cats and dogs with CIN were generally older than those without CIN. Nevertheless, the size of the whole wildcat kidneys (4–6cms long) obtained in our study would suggest that these animals were at least full-grown adults.
Kidney trace element profile does not suggest a causal link to CKD
Arsenic
Previous work by us had determined higher levels of total arsenic being measured in commercially available, complete pet foods containing fish30, which are more commonly fed to pet cats than to pet dogs. The evolutionary history of the domestic cat, originating from the Near Eastern desert region37, suggests that fish were never a common food source. Thus, the domestic cat may not be physiologically adapted to constituents found at high levels in fish, such as arsenic, mercury44 and iodine45,46. In the present study, we were unable to determine diet histories and thus were unable to associate fish intake with kidney tissue arsenic content. The higher levels of total arsenic in kidney tissue and urine in domestic cats compared to dogs and Scottish wildcats, likely reflect dietary intake over the previous few months and days, respectively prior to either the planned euthanasia or death (in case of wildcats). In addition, domestic cats and dogs could have reduced their intake in the days prior to euthanasia, which could influence (reduce) our urine, but not kidney, elemental levels. Nevertheless, the Scottish wildcat is unlikely to have ever consumed a commercial diet, nor any wild fish; instead obtaining its food by hunting wild rabbits, rodents and birds36, which may explain its lower renal accumulation of arsenic compared to the domestic cat.
The kidney excretes the majority of any absorbed arsenic in the urine within 24–48 hours of its consumption26,47,48, thus spot samples of urine collected at necropsy are likely to reflect arsenic intake over the few days prior to death, rather than being indicative of chronic intake. Greater urinary arsenic excretion has, however, been associated with increased dietary intake48 and an increased risk of renal dysfunction and CKD27. Indeed, in the current study, urinary arsenic concentrations were significantly greater in cats and dogs with CIN compared to those without CIN, suggesting an association with a risk factor for CKD in the companion animal population. However, we found no consistent stratified increase in kidney tissue arsenic content and stage of CIN. Kidney levels of arsenic were, in general, very low. However, while urinary arsenic concentrations have been measured in other species and has been associated with renal disease; to our knowledge, the kidney arsenic content was unknown. Therefore, conclusions on any relationship between increased intake of fish-based foods, longer-term arsenic ingestion and renal bioaccumulation with the development of CKD in the domestic cat population are not reliably supported by the current study. Arsenic accumulates primarily in the kidney but also in other organs (liver > pancreas > aorta > testis)20. Thus, our data do not exclude the possibility that renal tissue arsenic content biomarks ectopic accumulation. High arsenic intake, particularly of inorganic forms, is known to cause kidney disease and bladder cancer, often underpinned by extensive oxidative damage49,50,51.
Arsenic in fish-containing pet foods is primarily an organic, non-toxic form
High total arsenic concentration in fish-containing wet foods for cats, as determined by us previously30, is almost entirely organic AsB. Only minute amounts of DMA and MMA were observed. As(III) and As(V), both of which are highly toxic, were not-detectable in any food, similar to previously published studies on arsenic in fish for human consumption33. Thus, whilst it is acknowledged that some fish-based pet foods have higher than average levels of arsenic content, despite most being within legal limits, the type of arsenic species are predominantly organic forms that are unlikely to represent any biological risk to companion animals.
Iron
iron is a known catalyst in the formation of ROS52 and is therefore implicated in the development of oxidative stress which can promote renal injury53,54. Iron is not normally stored in the kidneys in great quantities and is only minimally excreted in urine since most circulating iron is bound to proteins such as the iron-transport protein, transferrin, and iron that does pass into the renal tubules is mostly reabsorbed55. Increased levels of iron have been found in the kidney tissue, associated with glomerular lesions56,57, and urine53,58 of human CKD patients compared to humans without renal disease. However, we measured lower concentrations of iron in cat, compared to dog, kidneys and, in both species, in kidneys with CIN compared to those without. Furthermore, we saw no difference in urinary iron concentrations between species or between healthy animals and those with CIN in our study.
Anti-oxidant minerals are low in cat versus dog kidneys
Copper and zinc
While elevated levels of pro-oxidant minerals increase the likelihood of oxidative damage, this phenomenon occurs due to an imbalance between these and the anti-oxidant defences that are in place within the tissues. Together, copper and zinc comprise the functional core of superoxide dismutase (SOD) isoforms (SOD1 and SOD3) – a potent antioxidant enzyme11. SOD1 is active in the cytosol while SOD3 activity occurs extracellularly59. The former constitutes 80% of the total SOD activity in the mammalian kidney60, thus the importance of copper and zinc is clear. Yet, in the present study, these minerals were both depleted in cat versus dog kidneys and copper was also decreased in kidneys with CIN compared to healthy kidneys. It is possible therefore that felids may have lower levels of SOD1 and SOD3 in their kidney per se, which could predispose them to oxidative damage which, over time, progresses toward chronic renal disease. Furthermore, a recent study showed that cats with CKD had lower levels of zinc stored in their kidneys compared to cats without renal disease61. However, the zinc content of kidney tissue in the current study did not differ with the presence or absence of CIN. Nonetheless, the lower levels of zinc stored in the cats’ kidneys, per se, may contribute to the cat’s propensity to develop CKD more readily than dogs.
Zinc is excreted primarily in faeces and only very small amounts are excreted into the urine by the kidney62. Thus, despite measuring greater zinc concentrations in dog, versus cat, urine, this may not reflect dietary concentrations of the mineral directly. However, in cats and dogs62 and rats63, kidney zinc levels have been shown to increase with increased dietary intake of this trace element. Another implication of our findings might therefore be that cats receive less zinc from their diet than dogs. Dietary histories were not obtained for the animals in our study and our previous work showed no significant difference in the zinc content of commercially-available, complete foods for cats and dogs30. Nonetheless, relatively lower zinc intake could lead to increased oxidative stress in tissues, while adequate and increased dietary zinc could have a protective effect, as has been demonstrated in kidneys of diabetic mice64. However, excess dietary zinc intake has been shown to reduce renal function, induce systemic hypertension and reduce blood flow65. Importantly, pet foods appear to comply with maximum and minimum recommended levels of zinc30.
Multi-elemental analysis reveals further mineral differences in domestic cat vs. dog vs. Scottish wildcat kidneys
In the current study, magnesium, calcium and phosphorus, strontium and rubidium were each significantly different between groups. Lower magnesium content of cat kidneys (cf. dogs) is of interest: reduced intake of magnesium has been associated with reduced renal function66, increased risk of CKD in cats67 and increased fibroblast growth factor 23 (FGF23)68. Higher FGF23 can induce renal interstitial fibrosis69. Low serum magnesium is associated with an increased risk of death in cats70. Equally, nephrocalcinosis – deposits of calcium and phosphate in the kidneys – can be both a consequence and cause of renal disease71. We found cat and dog kidneys with CIN had higher calcium content, but in no sample was deposition to an extent that crystals were evident. No a priori hypotheses in regard to the biological role or toxicity of rubidium and strontium were proposed. Rubidium tends to follow potassium levels in the kidney and, generally, higher levels are reno-protective72. We found urinary rubidium and strontium levels to trend higher in dogs, a species less prone to renal disease, than cats. Lower levels of these two elements in cat, compared to dog, kidneys may imply that the feline kidney is less protected, although this would require further validation in other studies. Nevertheless, it is notable that tissue Rb in Scottish wildcats was double that of domestic cats, perhaps indicative of higher potassium intake and greater reno-protection.
In conclusion, domesticated cats, but not wildcats, have a high incidence of CIN, as compared to domesticated dogs. Furthermore, domesticated cats, but not wildcats or domesticated dogs, tend to be fed more fish-based foods, which have higher arsenic content, although this is primarily of a non-toxic form. Whilst domesticated cats, compared to dogs, do have increased renal and urinary levels of arsenic, indicative of greater dietary intake, the absolute quantities are very low, do not stratify with severity of CIN and are therefore unlikely to underpin the high incidence of CKD in this species. Nevertheless, our complete mineral profiling of cat and dog kidneys does reveal significantly lower concentrations of antioxidant minerals, such as copper and zinc, or potentially renal-protective minerals, such as rubidium and strontium, in domesticated cats relative to dogs. CKD is an age-related disease and an extended exposure to a pro-oxidant renal environment, due to diet or genetics, may be contributory toward renal disease in the domestic cat.
Source: Ecology - nature.com


