Fairen, A. G. et al. Astrobiology through the ages of Mars: the study of terrestrial analogues to understand the habitability of Mars. Astrobiology 10, 821–843 (2010).
Pollard, W. H., Omelon, C., Andersen, D. T. & McKay, C. P. Perennial spring occurrence in the expedition fiord area of western Axel Heiberg Island, Canadian High Arctic. Can. J. Earth Sci. 36, 105–120 (1999).
Pollard, W. et al. Overview of analogue science activities at the McGill Arctic Research Station, Axel Heiberg Island. Can. High Arctic. Planet. Sp. Sci. 57, 646–659 (2009).
Carr, M. H. & Head, J. W. Geologic history of Mars. Earth Planet. Sci. Lett. 294, 185–203 (2010).
Warner, N. et al. Late Noachian to Hesperian climate change on Mars: evidence of episodic warming from transient crater lakes near ares vallis. J. Geophys. Res. E Planets 115, 9002 (2010).
Rapin, W. et al. An interval of high salinity in ancient Gale crater. Nat. Geosci. 12, 889–895 (2019).
Wanke, H., Bruckner, J., Dreibus, G., Rieder, R. & Ryabchikov, I. Chemical composition of rocks and soils at the Pathfinder site. Space Sci. Rev. 96, 317–330 (2001).
Clark, B. C. et al. Inorganic analyses of martian surface samples at the Viking landing sites. Science. 194, 1283–1288 (1976).
Connell-Cooper, C. O., Spray, J., Thompson, L. & Berger, J. A. APXS-derived chemistry of the Bagnold dune sands: comparisons with Gale crater soils and the global martian average: APXS—Bagnold Sands and Gale Soils. J. Geophys. Res. Planets 122, 1–21 (2017).
Gellert, R. et al. Chemistry of rocks and soils in Gusev crater from the alpha particle X-ray spectrometer. Science. 305, 829–832 (2014).
Ehlmann, B. L. & Edwards, C. S. Mineralogy of the martian surface. Annu. Rev. Earth Planet. Sci. 42, 291–315 (2014).
Burgess, R., Wright, I. P. & Pillinger, C. T. Distribution of sulphides and oxidised sulphur components in SNC meteorites. Earth Planet. Sci. Lett. 93, 314–320 (1989).
Gooding, J. L. Soil mineralogy and chemistry on Mars: possible clues from salts and clays in SNC meteorites. Icarus. 1, 28–41 (1992).
Burns, R. G. & Fisher, D. S. Evolution of sulfide mineralization on Mars. J. Geophys. Res. 95, 14169–14173 (1990).
Morris, R. V. et al. Iron mineralogy and aqueous alteration from Husband Hill through Home Plate at Gusev crater, Mars: results from the Mössbauer instrument on the Spirit Mars exploration rover. J. Geophys. Res. E Planets 113, 1 (2008).
Franz, H. B. et al. Large sulfur isotope fractionations in martian sediments at Gale crater. Nat. Geosci. 10, 658–662 (2017).
Martin, P. E. et al. A two-step K-Ar experiment on Mars: dating the diagenetic formation of jarosite from Amazonian groundwaters. J. Geophys. Res. Planets 122, 2803–2818 (2017).
Lasue, J., Clifford, S. M., Conway, S. J., Mangold, N. & Butcher, F. E. The hydrology of Mars including a potential cryosphere. in Filiberto, J., Schwenzer S. P., (Eds.), Volatiles in the Martian Crust 185–246 (2019).
Orosei, R. et al. Radar evidence of subglacial liquid water on Mars. Science. 361, 448–449 (2018).
Jannasch, H. W. The chemosynthetic support of life and the microbial diversity at deep-sea hydrothermal vents. Proc. R. Soc. 225, 277–297 (1985).
Visser, J. A. N. M., Robertson, L. A. & Verseveld, H. W. V. A. N. Sulfur production by obligately chemolithoautotrophic Thiobacillus species. Appl. Environ. Microbiol. 63, 2300–2305 (1997).
Meier, D. V. et al. Niche partitioning of diverse sulfur-oxidizing bacteria at hydrothermal vents. ISME J. 11, 1545–1558 (2017).
McNichol, J. et al. Primary productivity below the seafloor at deep-sea hot springs. Proc. Natl. Acad. Sci. 115, 6756–6761 (2018).
Fike, D. A., Bradley, A. S. & Rose, C. V. Rethinking the ancient sulfur cycle. Annu. Rev. Earth Planet. Sci. 43, 593–622 (2015).
Mahaffy, P. R. et al. Abundance and isotopic compoisition of gases in the martian atmosphere from the Curiosity rover. Science. 341, 263–266 (2013).
Stern, J. C. et al. Evidence for indigenous nitrogen in sedimentary and aeolian deposits from the Curiosity rover investigations at Gale crater, Mars. Proc. Natl. Acad. Sci. 112, 4245–4250 (2015).
Jakosky, B. M. et al. Mars’ atmospheric history derived from upper-atmosphere measurements of 38Ar/36Ar. Science. 355, 1408–1410 (2017).
Schwenzer, S. P. & Kring, D. A. Impact-generated hydrothermal systems capable of forming phyllosilicates on Noachian Mars. Geology 37, 1091–1094 (2009).
Schwenzer, S. P. et al. Fluids during diagenesis and sulfate vein formation in sediments at Gale crater. Mars. Meteorit. Planet. Sci. 51, 2175–2202 (2016).
McAdam, A. C., Zolotov, M. Y., Mironenko, M. V. & Sharp, T. G. Formation of silica by low-temperature acid alteration of martian rocks: Physical-chemical constraints. J. Geophys. Res. E Planets 113, 1–8 (2008).
Martín-Torres, F. J. et al. Transient liquid water and water activity at Gale crater on Mars. Nat. Geosci. 8, 357–361 (2015).
Fox-Powell, M. G., Hallsworth, J. E., Cousins, C. R. & Cockell, C. S. Ionic strength is a barrier to the habitability of Mars. Astrobiology 16, 427–442 (2016).
McEwen, A. S. et al. Supporting material for seasonal flows on warm martian slopes. Science 333, 740–743 (2011).
Soare, R., Pollard, W. & Green, D. Deductive model proposed for evaluating terrestrial analogues. Eos. 82, 501 (2001).
Malin, M. C. & Edgett, K. S. Evidence for recent groundwater seepage and surface runoff on Mars. Science. 288, 2330–2336 (2000).
Andersen, D. T. & Pollard, W. H. Cold springs in permafrost on Earth and Mars. J. Geophys. Res. 107, 5015 (2002).
Malin, M. C., Edgett, K. S., Posiolova, L. V., Mccolley, S. M. & Dobrea, E. Z. N. Present-day impact cratering rate and contemporary gully on Mars. Science. 314, 1573–1578 (2006).
Battler, M. M., Osinski, G. R. & Banerjee, N. R. Mineralogy of saline perennial cold springs on Axel Heiberg Island, Nunavut, Canada and implications for spring deposits on Mars. Icarus 224, 364–381 (2013).
Rossi, A. P. et al. Large-scale spring deposits on Mars? J. Geophys. Res. 113, 1–17 (2008).
Brown, R. J. E. Permafrost in the Canadian Arctic archipelago. Geomorphol. Suppl. 13, 102–130 (1972).
Pollard, W. H. Icing processes associated with high Arctic perennial springs, Axel Heiberg Island, Nunavut Canada. Permafr. Periglac. Process. 16, 51–68 (2005).
Omelon, C. R., Pollard, W. H. & Andersen, D. T. A geochemical evaluation of perennial spring activity and associated mineral precipitates at Expedition Fjord, Axel Heiberg Island. Can. High Arctic. Appl. Geochem. 21, 1–15 (2006).
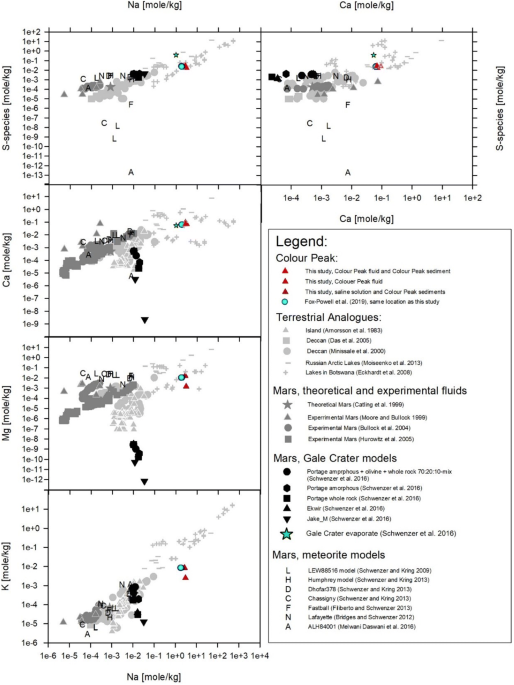
Fox-Powell, M. G. et al. Natural analogue constraints on Europa’s non-ice surface material. Geophys. Res. Lett. 46, 5759–5767 (2019).
Niederberger, T. D. et al. Novel sulfur-oxidizing streamers thriving in perennial cold saline springs of the Canadian High Arctic. Environ. Microbiol. 11, 616–629 (2009).
Perreault, N. N., Andersen, D. T., Pollard, W. H., Greer, C. W. & Whyte, L. G. Characterization of the prokaryotic diversity in cold saline perennial springs of the Canadian High Arctic. Appl. Environ. Microbiol. 73, 1532–1543 (2007).
Eckardt, F. D., Bryant, R. G., McCulloch, G., Spiro, B. & Wood, W. W. The hydrochemistry of a semi-arid pan basin case study: Sua Pan, Makgadikgadi, Botswana. Appl. Geochem. 23, 1563–1580 (2008).
Moiseenko, T. I., Gashkina, N. A., Dinu, M. I., Kremleva, T. A. & Khoroshavin, V. Y. Aquatic geochemistry of small lakes: effects of environment changes. Geochem. Int. 51, 1 (2013).
Minissale, A., National, I., Vaselli, O. & Chandrasekharam, D. Origin and evolution of `intracratonic’ thermal fluids from central-western peninsular India. Earth Planet. Sci. Lett. 181, 377–394 (2000).
Das, A., Rishnaswami, S. K., Arin, M. M. S. & Ande, K. P. Chemical weathering in the Krishna Basin and Western Ghats of the Deccan Traps India. Geochim. Cosmochim. Acta 69, 2067–2084 (2005).
Bullock, M. A., Moore, J. M. & Mellon, M. T. Laboratory simulations of Mars aqueous geochemistry. Icarus 170, 404–423 (2004).
Catling, D. C. A chemical model for evaporites on early Mars’ possible sedimentary tracers of the early climate and implications for exploration. J. Geophys. Res. 104, 16453–16469 (1999).
Moore, J. M. & Bullock, A. Experimental studies of Mars-analog brines. J. Geophys. Res. 104, 21925–21934 (1999).
Schwenzer, S. P. & Kring, D. A. Alteration minerals in impact-generated hydrothermal systems: exploring host rock variability. Icarus 226, 487–496 (2013).
Filiberto, J. et al. A review of volatiles in the martian interior. Meteorit. Planet. Sci. 1958, 1935–1958 (2016).
Melwani Daswani, M., Schwenzer, S. P., Reed, M. H., Wright, I. P. & Grady, M. M. Alteration minerals, fluids, and gases on early Mars: predictions from 1-D flow geochemical modeling of mineral assemblages in meteorite ALH 84001. Meteorit Planet Sci. 2174, 2154–2174 (2016).
Tosca, N. J., Mclennan, S. M., Lamb, M. P. & Grotzinger, J. P. Physicochemical properties of concentrated martian surface waters. J. Geophys. Res. 116, 1–16 (2011).
Bridges, J. C. & Schwenzer, S. P. The nakhlite hydrothermal brine on Mars. Earth Planet. Sci. Lett. 359–360, 117–123 (2012).
Niederberger, T. D. et al. Microbial characterization of a subzero, hypersaline methane seep in the Canadian High Arctic. ISME J. 4, 1326–1339 (2010).
Lay, C. Y. et al. Microbial diversity and activity in hypersaline High Arctic spring channels. Extremophiles 16, 177–191 (2012).
Lay, C. Y. et al. Defining the functional potential and active community members of a sediment microbial community in a high-arctic hypersaline subzero spring. Appl. Environ. Microbiol. 79, 3637–3648 (2013).
Lamarche-Gagnon, G., Comery, R., Greer, C. W. & Whyte, L. G. Evidence of in situ microbial activity and sulphidogenesis in perennially sub-0 °C and hypersaline sediments of a high Arctic permafrost spring. Extremophiles 19, 1–15 (2015).
Sapers, H. M. et al. Biological characterization of microenvironments in a hypersaline cold spring Mars analog. Front. Microbiol. 8, 2527 (2017).
Willerslev, E., Hansen, A. J. & Poinar, H. N. Isolation of nucleic acids and cultures from fossil ice and permafrost. Trends Ecol. Evol. 19, 141–147 (2004).
Willerslev, E. et al. Long-term persistence of bacterial DNA. Curr. Biol. 14, 13–14 (2004).
Pietramellara, G. et al. Extracellular DNA in soil and sediment: Fate and ecological relevance. Biol. Fertil. Soils 45, 219–235 (2009).
Li, R. et al. Comparison of DNA-, PMA-, and RNA-based 16S rRNA Illumina sequencing for detection of live bacteria in water. Sci. Rep. 7, 1–11 (2017).
Stamenković, V., Ward, L. M., Mischna, M. & Fischer, W. W. O2 solubility in martian near-surface environments and implications for aerobic life. Nat. Geosci. 11, 905–909 (2018).
Ventosa, A., Nieto, J. J. & Oren, A. Biology of moderately halophilic aerobic bacteria. Microbiol. Mol. Biol. Rev. 62, 504–544 (1998).
Kelly, D. P. Halothiobacillus. Bergey’s Man Syst. Archaea Bact. 1, 1–3. https://doi.org/10.1002/9781118960608.gbm01133 (2015).
Brinkhoff, T., Kuever, J., Muyzer, G. & Jannasch, H. W. Thiomicrospira. Bergey’s Man Syst. Archaea Bact 1, 1–10. https://doi.org/10.1002/9781118960608.gbm01221 (2015).
Kelly, D. P., Wood, A. P. & Stackebrandt, E. Thiobacillus. Bergey’s Man. Syst. Archaea Bact 1, 1–10. https://doi.org/10.1002/9781118960608.gbm00969 (2015).
Choi, B.-R. et al. Characterization of facultative sulfur-oxidizing Marinobacter sp. BR13 isolated from marine sediment of Yellow Sea, Korea. J. Korean Soc. Appl. Biol. Chem. 52, 309–314 (2009).
Sorokin, D. Y. Oxidation of inorganic sulfur compounds by obligately organotrophic bacteria. Microbiology 72, 641–653 (2003).
Bridges, J. C., Hicks, L. J. & Treiman, A. H. Carbonates on Mars. in Filiberto, J. Schwenzer S. P., (Eds.), Volatiles in the Martian Crust, pp. 89–118 (2019).
Franz, H. B., King, P. L. & Gaillard, F. Sulfur on Mars from the atmosphere to the core. in Filiberto, J. Schwenzer S. P., (Eds.), Volatiles in the Martian Crust, pp. 119–184 (2019).
Grotzinger, J. P. et al. A habitable fluvio-lacustrine environment at Yellowknife Bay, Gale Crater Mars. Science. 343, 1242777 (2014).
Fastook, J. L., Head, J. W., Marchant, D. R., Forget, F. & Madeleine, J. B. Early Mars climate near the Noachian-Hesperian boundary: Independent evidence for cold conditions from basal melting of the south polar ice sheet (Dorsa Argentea Formation) and implications for valley network formation. Icarus 219, 25–40 (2012).
Weiss, D. K. & Head, J. W. Crater degradation in the Noachian highlands of Mars: assessing the hypothesis of regional snow and ice deposits on a cold and icy early Mars. Planet. Space Sci. 117, 401–420 (2015).
Abramov, O. & Mojzsis, S. J. Thermal effects of impact bombardments on Noachian Mars. Earth Planet. Sci. Lett. 442, 108–120 (2016).
Fairén, A. G. A cold and wet Mars. Icarus 208, 165–175 (2010).
Perreault, N. N. et al. Heterotrophic and autotrophic microbial populations in cold perennial springs of the High Arctic. Appl. Environ. Microbiol. 74, 6898–6907 (2008).
Liu, C. et al. Marinobacter antarcticus sp. nov., a halotolerant bacterium isolated from Antarctic intertidal sandy sediment. Int. J. Syst. Evol. Microbiol. 62, 1838–1844 (2012).
Moghadam, M. S. et al. Isolation and genome sequencing of four Arctic marine Psychrobacter strains exhibiting multicopper oxidase activity. BMC Genomics 17, 1–14 (2016).
Bakermans, C. et al. Psychrobacter cryohalolentis sp. nov. and Psychrobacter arcticus sp. nov., isolated from Siberian permafrost. Int. J. Syst. Evol. Microbiol. 56, 1285–1291 (2006).
Antwis, R. E. et al. Fifty important research questions in microbial ecology. FEMS Microbiol. Ecol. 93, 1 (2017).
Sogin, M. L. et al. Microbial diversity in the deep sea and the underexplored ‘rare biosphere’. Proc. Natl. Acad. Sci. 103, 12115–12120 (2011).
Larsen, S., Nielsen, L. P. & Schramm, A. Cable bacteria associated with long-distance electron transport in New England salt marsh sediment. Environ. Microbiol. Rep. 7, 175–179 (2015).
Huber, B., Herzog, B., Drewes, J. E., Koch, K. & Müller, E. Characterization of sulfur oxidizing bacteria related to biogenic sulfuric acid corrosion in sludge digesters. BMC Microbiol. 1, 1–11. https://doi.org/10.1186/s12866-016-0767-7 (2016).
Krishnakumar, B. & Manilal, V. B. Bacterial oxidation of sulphide under denitrifying conditions. Biotechnol. Lett. 21, 437–440 (1999).
Price, A., Pearson, V. K., Schwenzer, S. P., Miot, J. & Olsson-Francis, K. Nitrate-dependent iron oxidation: a potential Mars metabolism. Front. Microbiol. 9, 1–15 (2018).
Blumenberg, M., Seifert, R., Petersen, S. & Michaelis, W. Biosignatures present in a hydrothermal massive sulfide from the Mid-Atlantic Ridge. Geobiology 5, 435–450 (2007).
Banfield, J. F., Moreau, J. W., Chan, C. S., Welch, S. A. & Little, B. Search for life on Mars. Astrobiology 1, 448–465 (2001).
Douglas, S. Mineralogical footprints of microbial life. Am. J. Sci. 305, 503–525 (2015).
Glamoclija, M. et al. Biosignatures and bacterial diversity in hydrothermal deposits of Solfatara Crater, Italy. Geomicrobiol. J. 21, 529–541 (2010).
Chan, C. S., Fakra, S. C., Emerson, D., Fleming, E. J. & Edwards, K. J. Lithotrophic iron-oxidizing bacteria produce organic stalks to control mineral growth: implications for biosignature formation. ISME J. 5, 717–727 (2010).
Taylor, C. D., Wirsen, C. O. & Gaill, F. Rapid microbial production of filamentous sulfur mats at hydrothermal vents. Appl. Environ. Microbiol. 65, 2253–2255 (1999).
Engel, A. S., Lichtenberg, H., Prange, A. & Hormes, J. Speciation of sulfur from filamentous microbial mats from sulfidic cave springs using X-ray absorption near-edge spectroscopy. FEMS Microbiol. Lett. 269, 54–62 (2007).
Seager, S., Schrenk, M. & Bains, W. An astrophysical view of Earth-based metabolic biosignature gases. Astrobiology 12, 62–82 (2012).
Pellerin, A. et al. Large sulfur isotope fractionation by bacterial sulfide oxidation. Sci. Adv. 5, 1–7 (2019).
Dobson, V. P., Vreeland, R. H. & Chester, W. Halomonas. Bergey’s Man. Syst. Archaea Bact. https://doi.org/10.1002/9781118960608.gbm01190 (2015).
Sutter, B. et al. The sample analysis at Mars (SAM) detections of CO2 and CO insedimentary material from Gale crater, Mars: implications for the presence of organic carbon and microbial habitability on Mars. in Proceedings of the AGU Fall Meeting (2016).
Chatzigiannidou, I., Props, R. & Boon, N. Drinking water bacterial communities exhibit specific and selective necrotrophic growth. NPJ Clean Water 1, 22 (2018).
An, D. et al. Metagenomics of hydrocarbon resource environments indicates aerobic taxa and genes to be unexpectedly common. Environ. Sci. Technol. 47, 10708–10717 (2013).
Schmidt, O., Hink, L., Horn, M. A. & Drake, H. L. Peat: home to novel syntrophic species that feed acetate- and hydrogen-scavenging methanogens. ISME J 1, 1–13. https://doi.org/10.1038/ismej.2015.256 (2016).
Timmers, P. H. A. et al. Metabolism and occurrence of methanogenic and sulfate-reducing syntrophic acetate oxidizing communities in haloalkaline environments. Front. Microbiol. 9, 1–18 (2018).
Tillett, D. & Neilan, B. A. Xanthogenate nucleic acid isolation from cultured and environmental cyanobacteria. J. Phycol. 36, 251–258 (2000).
Green, M. R. & Sambrook, J. Precipitation of DNA with ethanol. Cold Spring Harb. Protoc. 2016, 1116–1120 (2016).
Walters, W. A. et al. Improved bacterial 16S rRNA gene (V4 and V4–5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems 1, 1–10 (2012).
Boylen, E. et al. QIIME 2: reproducible, interactive, scalable, and extensible microbiome data science. PeerJ https://doi.org/10.7287/peerj.preprints.27295 (2018).
DeSantis, T. Z. et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72, 5069–5072 (2006).
McDonald, D. et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 6, 610–618 (2012).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Lane, D.J. 16S/23S rRNA Sequencing. in Nucleic Acid Techniques in Bacterial Systematics (ed. Stackebrandt, E. and Goodfellow, M.) 115–175 (John Wiley and Sons, 1991).
Hall, A. T. BioEdit: an important software for molecular biology. GERF Bull. Biosci. 2, 60–61 (2011).
Hall, T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. No. 41, 95–98 (1999).
Pruesse, E., Peplies, J. & Glöckner, F.O. SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 28, 1823–1829 (2012).
McCollom, T. M. Geochemical constraints on sources of metabolic energy for chemolithoautotrophy in ultramafic-hosted deep-sea hydrothermal systems. Astrobiology 7, 933–950 (2007).
Nier, A. & Mcelroy, M. B. Structure of the neutral upper atmosphere of Mars. Science. 194, 28–30 (1976).
Jakosky, B. M. & Shock, E. L. The biological potential of Mars, the early Earth, and Europa. J. Geophys. Res. E Planets 103, 19359–19364 (1998).
McCollom, T. M. & Amend, J. P. A thermodynamic assessment of energy requirements for biomass synthesis by chemolithoautotrophic micro-organisms in oxic and anoxic environments. Geobiology 3, 135–144 (2005).
Kaye, J. Z., Márquez, M. C., Ventosa, A. & Baross, J. A. Halomonas neptunia sp. nov., Halomonas sulfidaeris sp. nov., Halomonas axialensis sp. nov. and Halomonas hydrothermalis sp. nov.: halophilic bacteria isolated from deep-sea hydrothermal-vent environments. Int. J. Syst. Evol. Microbiol. 54, 499–511 (2004).
Lee, J. C. et al. Halomonas taeanensis sp. nov., a novel moderately halophilic bacterium isolated from a solar saltern in Korea. Int. J. Syst. Evol. Microbiol. 55, 2027–2032 (2005).
Yumoto, I. et al. Psychrobacter piscatorii sp. nov., a psychrotolerant bacterium exhibiting high catalase activity isolated from an oxidative environment. Int. J. Syst. Evol. Microbiol. 60, 205–208 (2010).
VanTrappen, S., Mergaert, J. & Swings, J. Loktanella salsilacus gen. nov., sp. Nov., Loktanella fryxellensis sp. Nov. and Loktanella vestfoldensis sp. Nov., new members of the Rhodobacter group isolated from microbial mats in Antarctic lakes. Int. J. Syst. Evol. Microbiol. 54, 1263–1269 (2004).
Hahnke, R. L., Meier-kolthoff, J. P. & García-lópez, M. Genome-based taxonomic classification of Bacteroidetes. Front. Microbiol. 7, 1–37 (2016).
Godoy, F. A., Swings, J. & Rehm, B. Sphingopyxis chilensis sp. Nov., a chlorophenol-degrading bacterium that accumulates polyhydroxyalkanoate, and transfer of Sphingomonas alaskensis to Sphingopyxis alaskensis comb. Nov.. Int J. Syst. Evol. Microbiol. 53, 473–477 (2003).
Yu, Y. et al. Sporosarcina antarctica sp. Nov., a psychrophilic bacterium isolated from the Antarctic. Int. J. Syst. Evol. Microbiol. 3, 2114–2117 (2008).
Satola, B., Wübbeler, J. H. & Steinbüchel, A. Metabolic characteristics of the species Variovorax paradoxus. Appl. Microbiol. Biotechnol. 97, 541–560 (2013).
Morohoshi, T. et al. Biofilm formation and degradation of commercially available biodegradable plastic films by bacterial consortiums in freshwater environments. Microbes Environ. 33, 332–335 (2018).
Glockner, F. O., Babenzien, H. & Amann, R. Phylogeny and identification in situ of Nevskia ramosa. Appl. Environ. Microbiol. 64, 1895–1901 (1998).
Sorokin, D. Y. et al. Discovery of extremely halophilic, methyl-reducing euryarchaea provides insights into the evolutionary origin of methanogenesis. Nat. Microbiol. 2, 1 (2017).
Reasoner, D. J. et al. A new medium for the enumeration and subculture of Bacteria from potable water. Appl. Environ. Microbiol. 49, 1–7 (1985).
Bertani, G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62, 293–300 (1951).
Macey, M. C., Pratscher, J., Crombie, A. & Murrell, J. C. Draft genome sequences of obligate methylotrophs Methylovorus sp. strain MM2 and Methylobacillus sp. Strain MM3, isolated from grassland soil. Microbiol. Resour. Announc. 7, 1–2 (2018).
Sievert, S. M., Heidorn, T. & Kuever, J. Halothiobacillus kellyi sp. Nov., a mesophilic, obligately chemolithoautotrophic, sulfur-oxidizing bacterium isolated from a shallow-water hydrothermal vent in the Aegean Sea, and emended description of the genus Halothiobacillus. Int. J. Syst. Evol. Microbiol. 50, 1229–1237 (2000).
Hobbs, G., Frazer, C. M., Gardner, D. C. J., Cullum, J. A. & Oliver, S. G. Dispersed growth of Streptomyces in liquid culture. Appl. Microbiol. Biotechnol. 31, 272–277 (1989).
Source: Ecology - nature.com


