Poiani, K. A., Richter, B. D., Anderson, M. G. & Richter, H. E. Biodiversity Conservation at Multiple Scales: Functional Sites, Landscapes, and Networks. BioScience 50, 133 (2000).
Hortal, J., Roura-Pascual, N., Sanders, N. J. & Rahbek, C. Understanding (insect) species distributions across spatial scales. Ecography 33, 51–53 (2010).
Kraft, N. J. B. et al. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 29, 592–599 (2015).
Keddy, P. A. Assembly and response rules: two goals for predictive community ecology. J. Veg. Sci. 3, 157–164 (1992).
Pärtel, M., Zobel, M., Zobel, K., van der Maarel, E. & Partel, M. The Species Pool and Its Relation to Species Richness: Evidence from Estonian Plant Communities. Oikos 75, 111–117 (1996).
Harrison, S. & Cornell, H. Toward a better understanding of the regional causes of local community richness. Ecol. Lett. 11, 969–979 (2008).
Cornwell, W. K. & Ackerly, D. D. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecol. Monogr. 79, 109–126 (2009).
Diaz, S., Cabido, M. & Casanoves, F. Plant functional traits and environmental filters at a regional scale. J. Veg. Sci. 9, 113–122 (1998).
Hawkins, B. A. et al. Energy, water and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117 (2003).
O’Brien, E. Water-energy dynamics, climate, and prediction of woody plant species richness: an interim general model. J. Biogeogr. 25, 379–398 (1998).
Dambros, C. S., Cáceres, N. C., Magnus, L. & Gotelli, N. J. Effects of neutrality, geometric constraints, climate, and habitat quality on species richness and composition of Atlantic Forest small-mammals: Distribution of small-mammal assemblages. Glob. Ecol. Biogeogr. 24, 1084–1093 (2015).
Kerr, J. T., Vincent, R. & Currie, D. J. Lepidopteran richness patterns in North America. Écoscience 5, 448–453 (1998).
O’Brien, E. M. Climatic Gradients in Woody Plant Species Richness: Towards an Explanation Based on an Analysis of Southern Africa’s Woody Flora. J. Biogeogr. 20, 181–198 (1993).
Clinebell, R. R., Phillips, O. L., Gentry, A. H., Stark, N. & Zuuring, H. Prediction of neotropical tree and liana species richness from soil and climatic data. Biodivers. Conserv. 4, 56–90 (1995).
Rahbek, C. & Graves, G. R. Multiscale assessment of patterns of avian species richness. Proc. Natl. Acad. Sci. 98, 4534–4539 (2001).
Laurance, W. F., Sayer, J. & Cassman, K. G. Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 29, 107–116 (2014).
Edwards, D. P., Gilroy, J. J., Thomas, G. H., Uribe, C. A. M. & Haugaasen, T. Land-Sparing Agriculture Best Protects Avian Phylogenetic Diversity. Curr. Biol. 25, 2384–2391 (2015).
Myers, J. A. & Harms, K. E. Seed arrival, ecological filters, and plant species richness: a meta-analysis. Ecol. Lett. 12, 1250–1260 (2009).
Mayfield, M. M. et al. What does species richness tell us about functional trait diversity? Predictions and evidence for responses of species and functional trait diversity to land-use change. Glob. Ecol. Biogeogr. 19, 423–431 (2010).
Filloy, J., Zurita, G. A. & Bellocq, M. I. Bird Diversity in Urban Ecosystems: The Role of the Biome and Land Use Along Urbanization Gradients. Ecosystems 22, 213–227 (2018).
Santoandré, S., Filloy, J., Zurita, G. A. & Bellocq, M. I. Ant taxonomic and functional diversity show differential response to plantation age in two contrasting biomes. For. Ecol. Manag. 437, 304–313 (2019).
Didham, R., Tylianakis, J., Gemmell, N., Rand, T. & Ewers, R. Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol. Evol. 22, 489–496 (2007).
Filloy, J., Zurita, G. A., Corbelli, J. M. & Bellocq, M. I. On the similarity among bird communities: Testing the influence of distance and land use. Acta Oecologica 36, 333–338 (2010).
Corbelli, J. M. et al. Integrating Taxonomic, Functional and Phylogenetic Beta Diversities: Interactive Effects with the Biome and Land Use across Taxa. Plos One 10, e0126854, https://doi.org/10.1371/journal.pone.0126854 (2015).
Zurita, G. A. & Bellocq, M. I. Bird Assemblages in Anthropogenic Habitats: Identifying a Suitability Gradient for Native Species in the Atlantic Forest. Biotropica 44, 412–419 (2012).
Normand, S. et al. Importance of abiotic stress as a range-limit determinant for European plants: insights from species responses to climatic gradients. Glob. Ecol. Biogeogr. 18, 437–449 (2009).
Bartlett, M. K., Scoffoni, C. & Sack, L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis: Drivers of plant drought tolerance. Ecol. Lett. 15, 393–405 (2012).
Spector, S. Scarabaeine Dung Beetles (coleoptera: Scarabaeidae: Scarabaeinae): An Invertebrate Focal Taxon for Biodiversity Research and Conservation. Coleopt. Bull. 60, 71–83 (2006).
Gardner, T. A., Hernández, M. I. M., Barlow, J. & Peres, C. A. Understanding the biodiversity consequences of habitat change: the value of secondary and plantation forests for neotropical dung beetles: Land-use change and tropical forest dung beetles. J. Appl. Ecol. 45, 883–893 (2008).
Barragán, F., Moreno, C. E., Escobar, F., Bueno-Villegas, J. & Halffter, G. The impact of grazing on dung beetle diversity depends on both biogeographical and ecological context. J. Biogeogr. 41, 1991–2002 (2014).
Gómez-Cifuentes, A., Munevar, A., Gimenez, V. C., Gatti, M. G. & Zurita, G. A. Influence of land use on the taxonomic and functional diversity of dung beetles (Coleoptera: Scarabaeinae) in the southern Atlantic forest of Argentina. J. Insect Conserv. 21, 147–156 (2017).
Alvarado, F., Escobar, F., Williams, D. R., Arroyo-Rodríguez, V. & Escobar-Hernández, F. The role of livestock intensification and landscape structure in maintaining tropical biodiversity. J. Appl. Ecol. 55, 185–194 (2018).
Halffter, G. & Arellano, L. Response of Dung Beetle Diversity to Human-Induced Changes in a Tropical Landscape. Biotropica 34, 144–154 (2002).
Alvarado, F. et al. Forest cover is more important than farmland heterogeneity and livestock intensification for the retention of dung beetle phylogenetic diversity. Ecol. Indic. 93, 524–532 (2018).
Giménez Gómez, V. C., Verdú, J. R., Guerra Alonso, C. B. & Zurita, G. A. Relationship between land uses and diversity of dung beetles (Coleoptera: Scarabaeinae) in the southern Atlantic forest of Argentina: which are the key factors? Biodivers. Conserv. 27, 3201–3213 (2018).
Gómez-Cifuentes, A., Giménez Gómez, V. C., Moreno, C. E. & Zurita, G. A. Tree retention in cattle ranching systems partially preserves dung beetle diversity and functional groups in the semideciduous Atlantic forest: The role of microclimate and soil conditions. Basic Appl. Ecol. 34, 64–74 (2019).
Guerra Alonso, C. B., Zurita, G. A. & Bellocq, M. I. Livestock areas with canopy cover sustain dung beetle diversity in the humid subtropical Chaco forest. Insect Conserv. Divers. 12, 296–308 (2019).
Davis, A. L. V., Scholtz, C. H. & Philips, T. K. Historical biogeography of scarabaeine dung beetles. J. Biogeogr. 29, 1217–1256 (2002).
Davis, A. J., Huijbregts, H. & Krikken, J. The role of local and regional processes in shaping dung beetle communities in tropical forest plantations in Borneo. Glob. Ecol. 9, 281–292 (2000).
Duncan, F. D. & Byrne, M. J. Discontinuous gas exchange in dung beetles: patterns and ecological implications. Oecologia 122, 452–458 (2000).
Nichols, E. et al. Global dung beetle response to tropical forest modification and fragmentation: A quantitative literature review and meta-analysis. Biol. Conserv. 137, 1–19 (2007).
Chown, S. L., Sørensen, J. G. & Terblanche, J. S. Water loss in insects: An environmental change perspective. J. Insect Physiol. 57, 1070–1084 (2011).
Chown, S. L. Physiological variation in insects: hierarchical levels and implications. J. Insect Physiol. 47, 649–660 (2001).
Gering, J. C., Crist, T. O. & Veech, J. A. Additive Partitioning of Species Diversity across Multiple Spatial Scales: Implications for Regional Conservation of Biodiversity. Conserv. Biol. 17, 488–499 (2003).
Lindenmayer, D. B., Franklin, J. F. & Fischer, J. General management principles and a checklist of strategies to guide forest biodiversity conservation. Biol. Conserv. 131, 433–445 (2006).
Cabeza, M. et al. Conservation planning with insects at three different spatial scales. Ecography 33, 54–63 (2010).
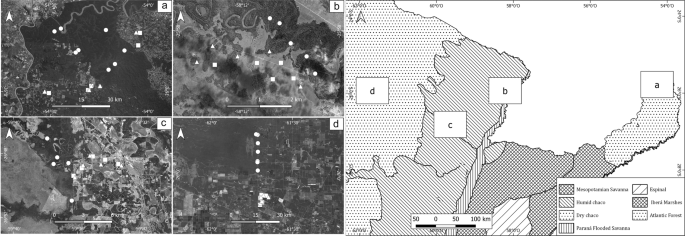
Prado, D. E. Seasonally dry forests of tropical South America: from forgotten ecosystems to a new phytogeographic unit. Edinb. J. Bot. 57, 437–461 (2000).
Cabrera, A. Enciclopedia Argentina de Agricultura y Ganaderia. Tomo II, (Acme, 1976).
Burkart, R., Barbaro, N. O., Sanchez, R. O. & Gomez, A. D. Eco-Regiones de la Argentina. (Presidencia de la Nación Secretaria de Recursos Naturales y Desarrollo Sustentable Administración de Parques Nacionales, 1999).
Oliveira-Filho, A. T. & Fontes, M. A. L. Patterns of Floristic Differentiation among Atlantic Forests in Southeastern Brazil and the Influence of Climate. 32, 793–810 (2000).
Brown, A. D. La situación ambiental Argentina 2005. (Fundación Vida Silvestre Argentina, 2006).
de Siqueira Neves, F. et al. Successional and Seasonal Changes in a Community of Dung Beetles (Coleoptera: Scarabaeinae) in a Brazilian Tropical Dry Forest. Nat. Conserv. 08, 160–164 (2010).
Larsen, T. H., Lopera, A. & Forsyth, A. Extreme Trophic and Habitat Specialization by Peruvian Dung Beetles (Coleoptera: Scarabaeidae: Scarabaeinae). Coleopt. Bull. 60, 315–324 (2006).
Salomão, R. P. & Iannuzzi, L. Dung beetle (Coleoptera, Scarabaeidae) assemblage of a highly fragmented landscape of Atlantic forest: from small to the largest fragments of northeastern Brazilian region. Rev. Bras. Entomol. 59, 126–131 (2015).
Vaz-de-Mello, F. Z. A multilingual key to the genera and subgenera of the subfamily Scarabaeinae of the New World (Coleoptera: Scarabaeidae). (Magnolia Press, 2011).
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas: new climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Cruaud, A. et al. Using insects to detect, monitor and predict the distribution of Xylella fastidiosa: a case study in Corsica. Sci. Rep. 8, (2018).
Chao, A., Chiu, C.-H. & Jost, L. Statistical challenges of evaluating diversity patterns across environmental gradients in mega-diverse communities. J. Veg. Sci. 27, 437–438 (2016).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Moran, P. A. P. Notes on continuous stochastic phenomena. Biometrika 37, 17–23 (1950).
Rangel, T. F., Diniz-Filho, J. A. F. & Bini, L. M. SAM: a comprehensive application for Spatial Analysis in Macroecology. Ecography 33, 46–50 (2010).
Zuur, A. F., Ieno, E. N. & Elphick, C. S. A protocol for data exploration to avoid common statistical problems: Data exploration. Methods Ecol. Evol. 1, 3–14 (2010).
Oksanen, J. et al. vegan: Community Ecology Package. (2017).
Peres-Neto, P. R., Legendre, P., Dray, S. & Borcard, D. Variation partitioning of species data matrices: Estimation and comparison of fractions. Ecology 87, 2614–2625 (2006).
Borcard, D., Gillet, F. & Legendre, P. Numerical ecology with R. (Springer, 2011).
Legendre, P. & Anderson, M. J. Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 69, 1–24 (1999).
Clarke, K. R. & Green, R. H. Statistical design and analysis for a ‘biological effects’ study. Mar. Ecol. Prog. Ser. 46, 213–226 (1988).
R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2017).
Cardoso, P., Rigal, F. & Carvalho, J. C. BAT – Biodiversity Assessment Tools, an R package for the measurement and estimation of alpha and beta taxon, phylogenetic and functional diversity. Methods Ecol. Evol. 6, 232–236 (2015).
Scheffler, P. Y. Dung beetle (Coleoptera: Scarabaeidae) diversity and community structure across three disturbance regimes in eastern Amazonia. J. Trop. Ecol. 21, 9–19 (2005).
Giraldo, C., Escobar, F., Chará, J. D. & Calle, Z. The adoption of silvopastoral systems promotes the recovery of ecological processes regulated by dung beetles in the Colombian Andes: Ecological processes regulated by dung beetles. Insect Conserv. Divers. 4, 115–122 (2011).
Escobar, F., Halffter, G. & Arellano, L. From forest to pasture: an evaluation of the influence of environment and biogeography on the structure of beetle (Scarabaeinae) assemblages along three altitudinal gradients in the Neotropical region. Ecography 30, 193–208 (2007).
Nichols, E. et al. Trait-dependent response of dung beetle populations to tropical forest conversion at local and regional scales. Ecology 94, 180–189 (2013).
Silva, P. Gda & Hernández, M. I. M. Local and Regional Effects on Community Structure of Dung Beetles in a Mainland-Island Scenario. Plos One 9, e111883 (2014).
Filgueiras, B. K. C. et al. Spatial replacement of dung beetles in edge-affected habitats: biotic homogenization or divergence in fragmented tropical forest landscapes? Divers. Distrib. 22, 400–409 (2016).
Damborsky, M. P., Alvarez Bohle, M. C., Ibarra Polesel, M. G., Porcel, E. A. & Fontana, J. L. Spatial and Temporal Variation of Dung Beetle Assemblages in a Fragmented Landscape at Eastern Humid Chaco. Neotrop. Entomol. 44, 30–39 (2015).
Verdú, J. R. et al. Grazing promotes dung beetle diversity in the xeric landscape of a Mexican Biosphere Reserve. Biol. Conserv. 140, 308–317 (2007).
Rös, M., Escobar, F. & Halffter, G. How dung beetles respond to a human-modified variegated landscape in Mexican cloud forest: a study of biodiversity integrating ecological and biogeographical perspectives: Dung beetle response to a human-modified variegated landscape. Divers. Distrib. 18, 377–389 (2012).
Moctezuma, V., Halffter, G. & Escobar, F. Response of copronecrophagous beetle communities to habitat disturbance in two mountains of the Mexican Transition Zone: influence of historical and ecological factors. J. Insect Conserv. 20, 945–956 (2016).
Liberal, C. N., Farias, Â. M. I., de, Meiado, M. V., Filgueiras, B. K. C. & Iannuzzi, L. How Habitat Change and Rainfall Affect Dung Beetle Diversity in Caatinga, a Brazilian Semi-Arid Ecosystem. J. Insect Sci. 11, 1–11 (2011).
Milchunas, D. G., Sala, O. G. & Lauenroth, W. K. A generalized model of the effects of grazing by large herbivores on grassland community structure.pdf. Am. Nat. 132, 87–106 (1988).
Quiroga, R. E., Golluscio, R. A., Blanco, L. J. & Fernández, R. J. F. Aridity and grazing as convergent selective forces: an experiment with an Arid Chaco bunchgrass. Ecol. Appl. 20, 14 (2010).
Larsen, T. H. Upslope Range Shifts of Andean Dung Beetles in Response to Deforestation: Compounding and Confounding Effects of Microclimatic Change: Dung Beetles Shift Upslope With Land-Use. Biotropica 44, 82–89 (2012).
Allen, A. P. & O’Connor, R. J. Interactive effects of land use and other factors on regional bird distributions. J. Biogeogr. 27, 889–900 (2000).
Duncan, R. P., Cassey, P. & Blackburn, T. M. Do climate envelope models transfer? A manipulative test using dung beetle introductions. Proc. R. Soc. B Biol. Sci. 276, 1449–1457 (2009).
Davis, A. L. V., Scholtz, C. H. & Deschodt, C. Multi-scale determinants of dung beetle assemblage structure across abiotic gradients of the Kalahari-Nama Karoo ecotone, South Africa. J. Biogeogr. 35, 1465–1480 (2008).
Liu, Y. et al. Functional beetle diversity in managed grasslands: effects of region, landscape context and land use intensity. Landsc. Ecol. 29, 529–540 (2014).
Jacobs, C. T., Scholtz, C. H., Escobar, F. & Davis, A. L. V. How might intensification of farming influence dung beetle diversity (Coleoptera: Scarabaeidae) in Maputo Special Reserve (Mozambique)? J. Insect Conserv. 14, 389–399 (2010).
Source: Ecology - nature.com


