Rohwer, F., Prangishvili, D. & Lindell, D. Roles of viruses in the environment. Environ. Microbiol. 11, 2771–2774 (2009).
Mauck, K. E., De Moraes, C. M. & Mescher, M. C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl Acad. Sci. USA 107, 3600–3605 (2010).
Ingwell, L. L., Eigenbrode, S. D. & Bosque-Pérez, N. A. Plant viruses alter insect behavior to enhance their spread. Sci. Rep. 2, https://doi.org/10.1038/srep00578 (2012).
Casteel, C. L. et al. The Niaro protein of Turnip mosaic virus improves growth and reproduction of the aphid vector, Myzus persicae (green peach aphid). Plant J. 77, 653–663 (2014).
Eigenbrode, S. D., Ding, H., Shiel, P. & Berger, P. H. Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proc. R. Soc. Lond. Ser. B 269, 455–460 (2002).
Poulin, R. Manipulation of host behaviour by parasites: a weakening paradigm?. Proc. R. Soc. Lond. Ser. B 267, 787–792 (2000).
Poulin, R. in Advances in the Study of Behavior (eds Brockmann, H. J. et al.), Ch. 5, 151–186 (Academic Press, Burlington, 2010).
Hughes, D. P. et al. Behavioral mechanisms and morphological symptoms of zombie ants dying from fungal infection. BMC Ecol. 11, 13 (2011).
Holt, R. D. & Dobson, A. P. in Disease Ecology: Community Structure and Pathogen Dynamics (eds Collinge, S. K. & Ra, C.) 6–27 (Oxford Univ. Press, Oxford, 2006).
Dunbar, H. E., Wilson, A. C., Ferguson, N. R. & Moran, N. A. Aphid thermal tolerance is governed by a point mutation in bacterial symbionts. PLoS Biol. 5, e96 (2007).
Parker, B. J. & Brisson, J. A. Laterally transferred viral gene modifies aphid wing plasticity. Curr. Biol. 29, 2098–2103.e5 (2019).
Hatcher, M. J., Dick, J. T. & Dunn, A. M. How parasites affect interactions between competitors and predators. Ecol. Lett. 9, 1253–1271 (2006).
Johnson, P. T. & Hoverman, J. T. Parasite diversity and coinfection determine pathogen infection success and host fitness. Proc. Natl Acad. Sci. USA 109, 9006–9011 (2012).
Lefèvre, T. et al. New prospects for research on manipulation of insect vectors by pathogens. PLoS Pathog. 2, e72 (2006).
Lefevre, T. & Thomas, F. Behind the scene, something else is pulling the strings: emphasizing parasitic manipulation in vector-borne diseases. Infect. Genet. Evol. 8, 504–519 (2008).
Stafford, C. A., Walker, G. P. & Ullman, D. E. Infection with a plant virus modifies vector feeding behavior. Proc. Natl Acad. Sci. USA 108, 9350–9355 (2011).
Koella, J. C., Rieu, L. & Paul, R. E. Stage-specific manipulation of a mosquito’s host-seeking behavior by the malaria parasite Plasmodium gallinaceum. Behav. Ecol. 13, 816–820 (2002).
Lee, S. J., Kang, D., Lee, S. C. & Ha, Y. R. Peculiar liquid-feeding and pathogen transmission behavior of Aedes togoi and comparison with Anopheles sinensis. Sci. Rep. 6, 20464 (2016).
De Moraes, C. M. et al. Malaria-induced changes in host odors enhance mosquito attraction. Proc. Natl. Acad. Sci. USA 111, 11079–11084 (2014).
De Boer, J. G. et al. Odours of Plasmodium falciparum-infected participants influence mosquito-host interactions. Sci. Rep. 7, 9283 (2017).
O’Shea, B. et al. Enhanced sandfly attraction toLeishmania-infected hosts. Trans. R. Soc. Trop. Med. Hyg. 96, 117–118 (2002).
Cornet, S., Nicot, A., Rivero, A. & Gandon, S. Malaria infection increases bird attractiveness to uninfected mosquitoes. Ecol. Lett. 16, 323–329 (2013).
Porras, M., De Moraes, C. M., Mescher, M. C., Rajotte, E. G. & Carlo, T. A. A plant virus (BYDV) promotes trophic facilitation in aphids on wheat. Sci. Rep. 8, 11709 (2018).
Poulin, R. “Adaptive” change in the behaviour of parasitized animals: a critical review. Int. J. Parasitol. 25, 1371–1383 (1995).
Anderson, R. M. & May, R. M. Infectious Diseases of Humans (Oxford Univ. Press, Oxford, 1991).
Márquez, L. M., Redman, R. S., Rodriguez, R. J. & Roossinck, M. A. Virus in a fungus in a plant: three-way symbiosis required for thermal tolerance. Science 315, 513–515 (2007).
Malmstrom, C. M., Melcher, U. & Bosque-Pérez, N. A. The expanding field of plant virus ecology: historical foundations, knowledge gaps, and research directions. Virus Res. 159, 84–94 (2011).
Xu, P. et al. Virus infection improves drought tolerance. N. Phytol. 180, 911–921 (2008).
Seabloom, E. W. et al. The community ecology of pathogens: coinfection, coexistence and community composition. Ecol. Lett. 18, 401–415 (2015).
Johnson, P. T., Preston, D. L., Hoverman, J. T. & LaFonte, B. E. Host and parasite diversity jointly control disease risk in complex communities. Proc. Natl Acad. Sci. USA 110, 16916–16921 (2013).
Hechinger, R. F. Parasites help find universal ecological rules. Proc. Natl Acad. Sci. USA 112, 1656–1657 (2015).
Holt, R. D. & Bonsall, M. B. Apparent competition. Annu. Rev. Ecol. Evol. Syst. 48, 447–471 (2017).
Memmott, J., Martinez, N. D. & Cohen, J. E. Predators, parasitoids and pathogens: species richness, trophic generality and body sizes in a natural food web. J. Anim. Ecol. 69, 1–15 (2000).
Ba-Angood, S. A. & Stewart, R. K. Occurrence, development, and distribution of cereal aphids on early and late cultivars of wheat, barley, and oats in southwestern Quebec. Can. Entomol. 112, 615–620 (1980).
Barro, P. J. & Wallwork, H. The role of annual grasses in the phenology of Rhopalosiphum padi in the low rainfall belt of South Australia. Ann. Appl. Biol. 121, 455–467 (1992).
Harrington, R. et al. Environmental change and the phenology of European aphids. Glob. Change Biol. 13, 1550–1564 (2007).
Dixon, A. F. G Aphid Ecology: An Optimization Approach (Springer, Netherlands, 2012).
Inbar, M. & Wool, D. Phloem-feeding specialists sharing a host tree: resource partitioning minimizes interference competition among galling aphid species. Oikos 73, 109–119 (1995).
Mei, H. K., Ming, C. C. & Jen, J. P. Temperature effects on life history traits of the corn leaf aphid, Rhopalosiphum maidis (Homoptera: Aphididae) on corn in Taiwan. Appl. Entomol. Zool. 41, 71–177 (2006).
Valenzuela, I. A Molecular Analysis of Aphids (Hemiptera: Aphididae) in South Eastern Australia. Ph.D thesis, The University of Melbourne (2008).
MacArthur, R. & Levins, R. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 101, 377–385 (1967).
Tilman, D. Resource Competition and Community Structure (Princeton Univ. Press, Princeton, 1982).
Magnuson, J. J., Crowder, L. B. & Medvick, P. A. Temperature as an ecological resource. Am. Zool. 19, 331–343 (1979).
Angilleta, M. Thermal Adaptation: A Theoretical and Empirical Synthesis (Oxford Univ. Press, Oxford, 2009).
Caillon, R., Suppo, C., Casas, J., Woods, H. & Pincebourde, S. Warming decreases thermal heterogeneity of leaf surfaces: implications for behavioural thermoregulation by arthropods. Funct. Ecol. 28, 1449–1458 (2014).
Ma, G. & Ma, C. S. Effect of acclimation on heat-escape temperatures of two aphid species: implications for estimating behavioral response of insects to climate warming. J. Insect Physiol. 58, 303–309 (2012).
Ma, G. & Ma, C. S. Climate warming may increase aphids’ dropping probabilities in response to high temperatures. J. Insect Physiol. 58, 1456–1462 (2012).
Pusag, J. C. A., Jahan, S. H., Lee, K. S., Lee, S. & Lee, K. Y. Upregulation of temperature susceptibility in Bemisia tabaci upon acquisition of Tomato yellow leaf curl virus (TYLCV). J. Insect Physiol. 58, 1343–1348 (2012).
Xu, D., Zhong, T., Feng, W. & Zhou, G. Tolerance and responsive gene expression of Sogatella furcifera under extreme temperature stresses are altered by its vectored plant virus. Sci. Rep. 6, 31521 (2016).
Habourdin, C., Klein, G., Araki, T., Williams, J. G. & Aubry, L. The arrestin-domain containing protein AdcA is a response element to stress. Cell Commun. Signal. 11, 91–15 (2013).
Zhang, F., Zhao, Y., Chao, Y., Muir, K. & Han, Z. Cubilin and amnionless mediate protein reabsorption in Drosophila nephrocytes. J. Am. Soc. Nephrol. 24, 209–216 (2013).
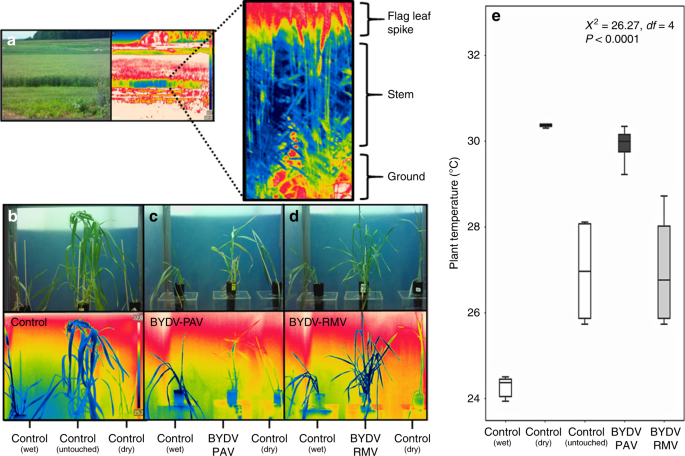
Chaerle, L. et al. Presymptomatic visualization of plant-virus interactions by thermography. Nat. Biotechnol. 17, 813–816 (1999).
Miller, W. A. & Rasochová, L. Barley yellow dwarf viruses. Annu. Rev. Phytopathol. 35, 167–190 (1997).
Hochachka, P. W. & Somero, G. N. Strategies of Biochemical Adaptation (WB Saunders, Philadelphia, 1973).
Sørensen, J. G., Kristensen, T. N. & Loeschcke, V. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 6, 1025–1037 (2003).
Van den Heuvel, J. F. et al. The N-terminal region of the luteovirus read through domain determines virus binding to Buchnera GroEL and is essential for virus persistence in the aphid. J. Virol. 71, 7258–7265 (1997).
Gray, S. M., Power, A. G., Smith, D. M., Seaman, A. J. & Altman, N. S. Aphid transmission of barley yellow dwarf virus: Acquisition access periods and virus concentration requirements. Phytopathology 81, 539–545 (1991).
Jiménez-Martínez, E. S., Bosque-Pérez, N. A., Berger, P. H. & Zemetra, R. S. Life history of the bird cherry-oat aphid, Rhopalosiphum padi (Homoptera: Aphididae), on transgenic and untransformed wheat challenged with barley yellow dwarf virus. J. Econ. Entomol. 97, 203–212 (2004).
Leinonen, I. & Jones, H. G. Combining thermal and visible imagery for estimating canopy temperature and identifying plant stress. J. Exp. Bot. 55, 1423–1431 (2004).
Ribeiro, P. L., Camacho, A. & Navas, C. A. Considerations for assessing maximum critical temperatures in small ectothermic animals: Insights from leaf-cutting ants. PLoS ONE 7, e32083 (2012).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Trapnell, C., Lior, P. & Steven, L. S. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 (2009).
Goff, L. A., Trapnell, C. & Kelley, D. CummeRbund: Visualization and Exploration of Cufflinks High-Throughput Sequencing Data. R Package Version 2.2.0 (2012).
Fussnecker, B. L., McKenzie, A. M. & Grozinger, C. M. cGMP modulates responses to queen mandibular pheromone in worker honey bees. J. Comp. Physiol. A 197, 939–948 (2011).
Hellerstein, D. & Mendelsohn, R. A theoretical foundation for count data models. Am. J. Agric. Econ. 75, 604–611 (1993).
Source: Ecology - nature.com


