RYGB surgery induced significant weight loss
We studied the microbiome and metabolome of two cohorts: longitudinal and cross-sectional populations. For the longitudinal arm of the study, we recruited nine morbidly obese pre-RYGB participants (a tenth participant dropped out after baseline measurements) and monitored their weight loss and health outcomes 6 months (RYGB-6_mo) and 12 months (RYGB-12_mo) after RYGB surgery. The study design is presented in Fig. 1a, and participant characteristics are summarized in Table 1. We also compared this longitudinal population to a previously studied cross-sectional RYGB cohort (RYGB-CS) (n = 24)6.
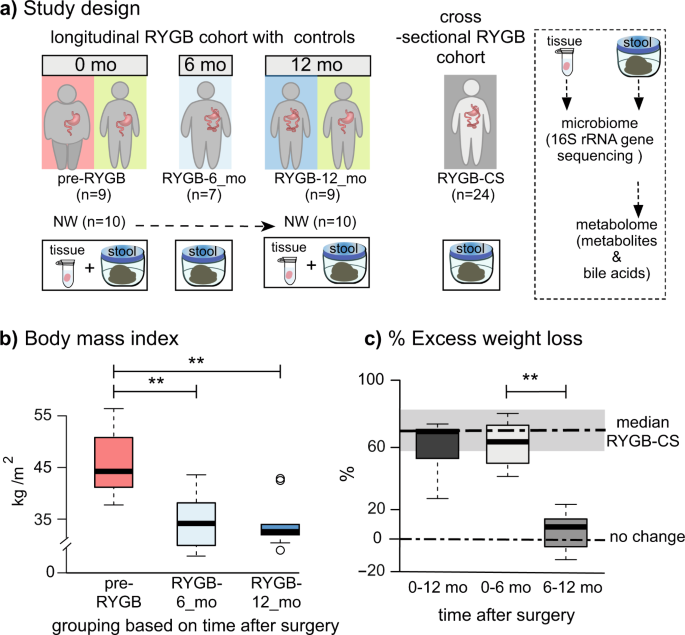
a Study design including number of participants and sample types collected longitudinally and cross-sectionally. b Body mass index (BMI) index of participants before the surgery (pre-RYGB), 6 months (RYGB-6_mo), and 12 months (RYGB-12_mo) after the surgery. c % Excess weight loss 12 months after the surgery, 6 months after the surgery, and 6–12 months after the surgery. The box plots represent minimum, maximum, median, first quartile and third quartile values. The gray shaded box around median of RYGB-CS represents median absolute deviation. Statistical significance between the groups was tested with Wilcoxon signed-rank test and p values were corrected using the Bonferroni method. **p < 0.01.
Figure 1b, c shows the short- and longer-term effects of RYGB surgery on weight loss. Percent excess weight loss (%EWL) calculations, also shown in Fig. 1c, confirm that the participants achieved the greatest weight loss during the initial 6 months and maintained the weight loss a year after the surgery. Median %EWL after 12 months (65 ± 10) was slightly lower than the median %EWL of the cross-sectional group (RYGB-CS) (73 ± 15)6; however, this difference was not statistically significant (p = 0.24).
Changes in participants’ diet regimens may have contributed to weight loss after the surgery. It is important to note that dietary intake survey was self-administered; hence, errors in completion could have occurred. Table 2 summarizes total dietary calories and dietary composition. Based on total calories reported, the morbidly obese participants (pre-RYGB) were consuming fewer calories than the normal weight (NW) participants. In the United States, it is often recommended to have morbidly obese patients lose 10% of their excess weight prior to surgery in order to minimize surgical complications1, even though pre-surgical weight loss has not been associated with a reduction in post-operative complications28. Our pre-RYGB participants were enrolled in a pre-surgery diet program, and according to the self-reported surveys, they appear to have restricted their calorie intake to achieve pre-surgical weight loss. Although the caloric intake increased by 22% at 12 months compared to 6 months after RYGB, the weight loss benefits were sustained. The dietary composition of the morbidly obese participants did not significantly change after the surgery (Wilcoxon signed-rank test, p = 0.683), although, compared to NW individuals, carbohydrates formed a smaller fraction of the diets of post-RYGB participants (Table 2). Our study results are consistent with prior reports that RYGB results in significant weight loss, especially during the first six months after the surgery29, and remain stable or continue to improve until up to one year after the surgery30.
Besides weight loss, RYGB is known to lead to resolution of many metabolic disorders, including Type II diabetes. At their baseline measurements, seven participants had high blood pressure and diabetes, eight of them had hyperlipidemia, and nine of them had degenerative osteoarthritis. After RYGB, a majority of the study participants had resolution of diabetes, hyperlipidemia, and hypertension (Table 1), but not arthritis. The metabolic improvements after RYGB are well known and our observations are in agreement with previous reports31,32.
RYGB altered fecal and mucosal microbiome structures
To detect changes in the gut microbiome after RYGB surgery, we analyzed the structure of fecal and mucosa-associated (mucosal) microbiomes of morbidly obese individuals (n = 9) before and after surgery. Rectal mucosal samples were collected at baseline and 12 months after RYGB via unsedated flexible sigmoidoscopy. Microbial DNA was extracted from fecal and mucosal samples. We performed weighted and unweighted Unifrac33 analyses on 16S rRNA gene sequences using the QIIME 1.9 suite34, and the principal component analyses (PCoA) are shown in Fig. 2. The effects of RYGB on the microbiome were pronounced for mucosal and fecal communities (Fig. 2a, b) for PCoA analysis based on unweighted Unifrac distances. As demonstrated by Fig. 2a, mucosal communities differed significantly on the PCo1 axis when comparing pre-RYGB group to RYGB-12_mo group (p = 0.02). Additionally, the pre-RYGB group was significantly different than the NW group, particularly on the PCo1 axis, indicating that microbiomes of normal weight and morbidly obese individuals differed in structure. Although PCoA based on unweighted Unifrac distances demonstrated the impact of RYGB on mucosal and luminal communities, PCo1 and PCo2 explained only a fraction (up to 13%) of the variability in the data set. Even though we controlled for factors such as, age of the participants and use of pharmaceuticals, heterogeneity in the human population, and other factors that influence gut microbiota composition led to a small fraction of variability in the data set being explained by the PCo1 and PCo2.
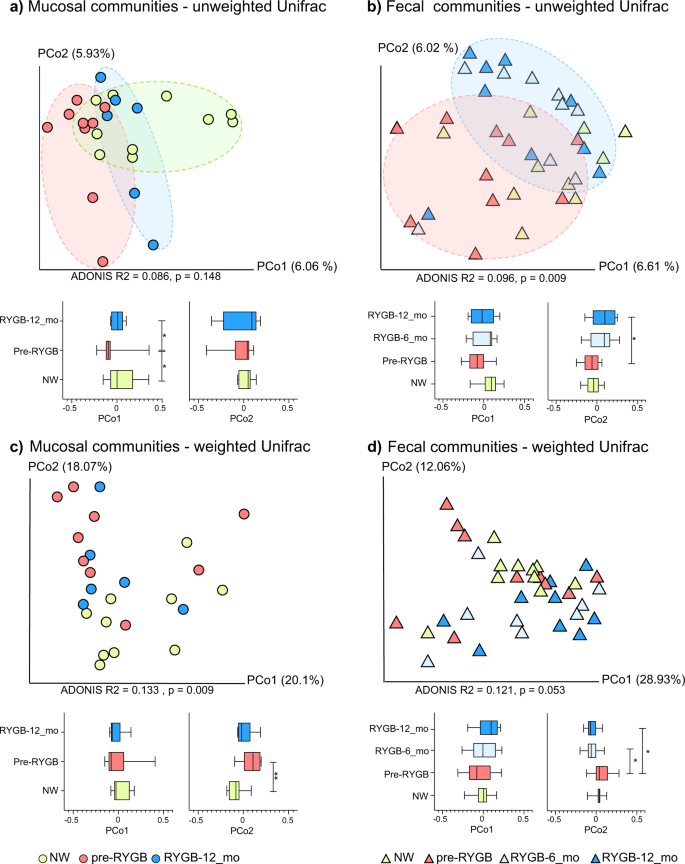
Microbiome communities (a mucosal) and (b fecal) before and after RYGB surgery in comparison to NW controls based on unweighted Unifrac distances. Microbiome communities (c mucosal) and (d fecal) before and after RYGB surgery in comparison to NW controls based on weighted Unifrac distances. Box plots represent the median distances among the communities on PCo1 and PCo2. * indicates Mann–Whitney U-test p < 0.05 and ** indicates Mann–Whitney U-test p < 0.01.
Differences in mucosal communities before and after RYGB were less apparent when weighted Unifrac (Fig. 2c) was used to calculate the dissimilarities among the communities. With weighted Unifrac analysis, the differences for minor taxa were obscured by the great abundances of Firmicutes and Bacteroidetes phylotypes.
Based on unweighted and weighted Unifrac distances, changes in the fecal microbiome appeared as soon as 6 months after the surgery, and the difference on PCo2 between pre-RYGB and RYGB-12_mo was significant (p = 0.04) (Fig. 2b, d). The ADONIS test was used on Unifrac distance matrices to differentiate overall differences in microbiome structure based on defined groups. The ADONIS R2 values ranged from 0.086 to 0.133 (p < 0.05) based on participant groups (NW, Pre-RYGB, RYGB-6_mo, and RYGB-12_mo) (Fig. 2). Even though these values are relatively small in terms of explaining the variation in the data set, ADONIS R2 values were smaller than 0.03 when grouping was based on gender, diet, BMI, stool consistency, or age. The ADONIS R2 results illustrate that our data set had high heterogeneity and variability; nevertheless, bariatric surgery had significantly greater impact on the overall microbiome structure than any of the other factors that commonly explain interpersonal variability, including diet and BMI.
When the RYGB-CS samples were incorporated into the weighted and unweighted Unifrac analysis, both RYGB-6_mo and RYGB-12_mo samples clustered together with RYGB-CS samples for weighted and unweighted Unifrac, although more strongly with the unweighted Unifrac (Supplementary Fig. 1, ADONIS R2 = 0.2401, p = 0.003). Additionally, when fecal and mucosal samples were analyzed together (Supplementary Fig. 1), clustering based primarily on sample type followed by the participant groups was observed, especially based on unweighted Unifrac distances. Interestingly, some of the RYGB-12_mo mucosal samples clustered with the fecal samples, indicating that after RYGB the mucosal community structure was more similar to the fecal community structure; however, the small sample size did not allow us to assess the significance of this observation. In summary, the results for the fecal microbiome are consistent with previous reports4,7,10,12 showing that fecal microbiome structure changed after RYGB, with changes sustained at least 1 year after surgery.
It is imperative to characterize changes in the microbiome of the mucosal space and the feces due to their differences and physiological relevance. In the lumen, substrates are usually dietary molecules, whereas in mucosal surfaces, they are host-derived glycans35. Another difference is the electron acceptor at the mucosal surfaces versus the lumen36. Oxygen derived from the eukaryotic tissues is gradually depleted in the mucosal layer by facultative anaerobes, and, therefore, the lumen becomes anaerobic18. Microorganisms that live in the lumen are also affected by other host-associated factors such as transit time, frequency and composition of dietary intake, and bile acids37.
Fecal microbiome after RYGB was similar to microbiome from a RYGB-CS cohort
Figure 3a shows the relative abundances of significantly enriched or depleted genus-level phylotypes in the lumen 6 months and 1 year after RYGB surgery and in comparison to a cross-sectional cohort (RYGB-CS). The surgery significantly altered relative abundances of 24 genus-level phylotypes (Wilcoxon signed-rank test p < 0.05). The majority of enrichments or depletions of genus-level phylotypes occurred within the first 6 months after surgery and were sustained 1 year after surgery (Fig. 3a and Supplementary Fig. 2). The abundances of these phylotypes were significantly different in RYGB-6_mo and RYGB-12_mo groups, compared to the NW group (Supplementary Fig. 2) (Mann–Whitney U-test p < 0.05). One of the microbial staples of RYGB surgery is enrichment of phylotypes from Gammaproteobacteria4,7, and our analysis showed that RYGB also altered the abundance of genus-level phylotypes from other phyla, including Firmicutes, Actinobacteria, Fusobacteria, and Bacteroidetes.
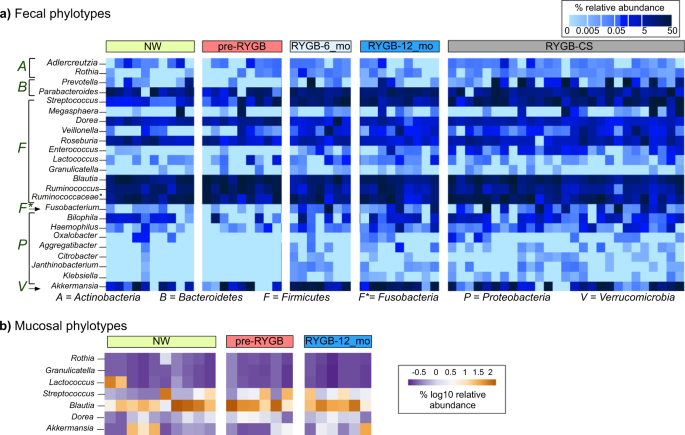
Heat map showing which a fecal and b mucosal microbial phylotypes were enriched or depleted after RYGB surgery. The samples were chronologically ordered based on time after surgery. The statistical significance between pre-RYGB and RYGB-12_mo groups were based on Wilcoxon signed-rank test and p values were corrected using Bonferroni method *p < 0.05.
We observed an increase in the abundance of Proteobacteria phylotypes Rothia, Aggregatibacter, Granulicatella, Citrobacter, Janthinobacterium, and Klebsiella. Firmicutes had many phylotypes whose relative abundances were affected by the surgery. While many phylotypes—such as Streptococcus, Enterococcus, Lactococcus, Veillonella, and Granulicatella—were enriched, Ruminococcus, Blautia, and Roseburia were depleted after the surgery. Akkermansia from Verrucomicrobia and Adlercruetzia and Rothia from Actinobacteria also were in greater abundance after RYGB.
Figure 3a also shows the relative abundance of the aforementioned phylotypes compared to a RYGB-CS group. The RYGB-CS group consisted of participants who had previously undergone RYGB, had lost at least 50% of their excess weight, and who had provided a stool sample 13–60 months after surgery; therefore, it was a more heterogeneous group by time after surgery6. The results seen in the RYGB-CS group paralleled those seen in the RYGB-6_mo and RYGB-12_mo groups (Supplementary Fig. 2). The results of the RYGB-CS group were sorted based on the time after their surgery, and we found no clustering based on time after surgery. These results support that changes in the microbiome occurred quickly after the surgery (within 6 months) and persisted in the long term (>60 months).
To confirm observations on genus-level phylotypes, we performed unsupervised clustering and generated hierarchical clustering heat map based on Euclidean distances among the samples (Supplementary Fig 3). Samples formed five distinct clusters driven by Fusobacterium, Prevotella, Ruminococcus, Parabacteroides, Blautia, and Akkermansia. Three of the clusters were composed of only post-RYGB samples (RYGB-6_mo, RYGB_12-mo, and RYGB-CS), indicating the impact of the RYGB alone on the relative abundance of genus-level phylotypes. The sustained changes observed in the microbiome after RYGB indicate that the surgery-imposed changes to the gut environment/ecosystem were persistent and permanently affected gut microbiota in a stronger way than interpersonal variations.
RYGB altered mucosal microbial communities increasing Akkermansia sp. and lactate metabolizers
Our analysis of enriched or depleted phylotypes also demonstrates that RYGB surgery led to a wide spectrum of changes in the mucosal space (Fig. 3b). Six genus-level phylotypes were significantly enriched in the mucosa after RYGB surgery: Granulicatella, Lactococcus, Streptococcus, Blautia, Dorea, and Akkermansia (Supplementary Fig. 4) (Wilcoxon signed-rank test p < 0.05). Relative abundances of these phylotypes also were greater in the NW mucosa, compared to the pre-RYGB mucosa. Except for Akkermansia, the microorganisms enriched post-surgery are from the Firmicutes phylum and are known to form biofilms and contribute to lactate metabolism38. Lactococcus, Streptococcus, and Granulicatella are lactate-producing microorganisms, whereas Dorea and Blautia are lactate oxidizers39. Lactate-producing Streptococcus and Lactococcus species have been used as probiotics to enhance gut epithelial barrier and integrity40, since lactate availability is crucial for butyrate producers and, therefore, colon epithelium health41.
In addition to lactate producers and oxidizers, we observed an increase in the relative abundance of Akkermansia in the mucosa after RYGB surgery. Previously, a similar trend was observed in mice after RYGB22, and our findings confirmed this observation in humans. Animal models have demonstrated that a weak gut barrier contributes to the development of endotoxemia and inflammation, which subsequently leads to insulin resistance and an increase in adiposity42,43,44. Akkermansia is a known mucin degrader, and its presence has been shown to improve the gut epithelial barrier, reduce adiposity of the organs, and protect against insulin resistance and obesity in humans45. However, a recent study that investigated the link between Akkermansia abundance in the feces of severely obese individuals after RYGB and diabetes did not report any association between Akkermansia abundance and glucose homeostasis after RYGB46. Overall, our results indicate that alterations in the gastrointestinal mucosa after RYGB may contribute to an increase in mucin-degrading, lactate-producing, and lactate-oxidizing microorganisms.
Post-RYGB microbiota alters the fecal metabolome
Changes in the structure of the gut microbiome after RYGB surgery were reflected in the gut metabolome. Figure 4 shows PCA results for the fecal metabolomes detected by gas chromatography-mass spectrometry (GC-MS) and 1H-NMR-based methods. Fecal water-soluble extracts were analyzed with 1H-NMR, while lyophilized fecal matter was analyzed with GC-MS. 1H-NMR provided mainly volatile and water-soluble compounds, whereas GC-MS identified many metabolites of the undigested nutrients and components of microbial cells.
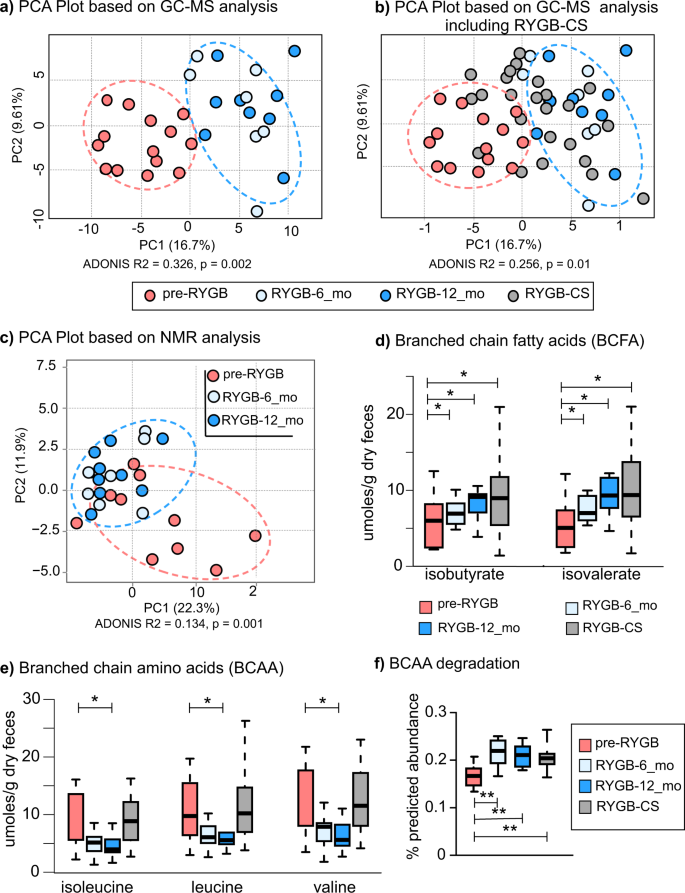
Principal component analysis based on metabolites detected by a GC-MS based metabolomes before and after the surgery. b Principal component analysis based on GC-MS metabolome that includes RYGB-CS group samples. c 1H-NMR based metabolomes before and after the surgery. d Branched-chain fatty acids—isobutyrate and isovalerate—measured with NMR after RYGB surgery prospectively and retrospectively. e Concentrations of isoleucine, leucine, and valine, branched-chain amino acids (BCAA) measured with NMR after RYGB surgery prospectively and retrospectively. The box plots represent minimum, maximum, median, first quartile and third quartile values. f Predicted relative abundance of the genes that are involved in branched chain amino acid (leucine, valine, and isoleucine) degradation. KEGG ko00280 valine, leucine, isoleucine degradation pathway was used in the analysis. * indicates statistical significance between pre-RYGB and RYGB-12_mo groups based on Wilcoxon signed-rank test and p values were corrected using Bonferroni method *p < 0.05.
Based on GC-MS data (Fig. 4a), RYGB-6_mo and RYGB-12_mo fecal metabolomes clustered away from pre-RYGB metabolomes (ADONIS R2 = 0.326, p = 0.002). Figure 4b overlays the GC-MS-based metabolomes of the RYGB-CS participants to the metabolomes of longitudinal study participants illustrated in Fig. 4a. The metabolomes of most of the RYGB-CS participants were more similar to RYGB-6_mo and RYGB-12_mo participants (Mann–Whitney U-test p < 0.05, ADONIS R2 = 0.256, p = 0.01) than to Pre-RYGB or NW participants, supporting the observation that the impact of RYGB surgery resulted in a unique metabolic fingerprint that was preserved in the long term. Moreover, the similar clustering patterns with the metabolome (Fig. 4a, b) and the microbiome (Fig. 2a) strengthen the conclusion that the changes in the microbiome and metabolome after RYGB surgery were linked, persistent, and stronger than interpersonal variations.
Similar to GC-MS metabolome, 1H-NMR quantification of water-soluble metabolites showed a distinct RYGB metabolome (Fig. 4c). The concentrations as measured by 1H-NMR of the major SCFAs of the human gut—acetate, butyrate, and propionate (Table 3)—were similar for the RYGB-CS and RYGB-12_mo groups. Propionate-to-acetate and butyrate-to-acetate ratios increased 6 and 12 months after the surgery, and the difference between baseline and 12-month samples was statistically significant (p = 0.03). We previously reported a similar trend with our RYGB-CS cohort6. Higher butyrate- and propionate-to-acetate ratios after the surgery compared to baseline indicates a shift in microbial metabolism from acetate production to butyrate and propionate production. Butyrate and propionate have been shown to signal free fatty acid receptors and induce a satiety response in the brain of mice47. Shifts in microbial metabolism reflect another potential mechanism explaining how microorganisms contribute to weight loss following RYGB.
We also evaluated the concentrations of branched chain amino acids (BCAA) and their fermentation products—branched-chain fatty acids (BCFA)—before and after RYGB. As seen in Fig. 4d, the fecal concentrations of two BCFAs—isobutyrate and isovalerate—increased after surgery and this observation is consistent with previous observations8,13. The RYGB-CS and RYGB-12_mo groups had similar concentrations of these BCFAs. Therefore, we can deduce that an increase in the abundance of these BCFAs was more likely associated with RYGB. Three BCAAs—leucine, isoleucine, and valine—were at significantly lower abundance in RYGB-12_mo in comparison to NW and RYGB-CS groups (Fig. 4e, Table S1). Interestingly, the concentration of BCAAs poorly correlated with the amount of protein consumed by the participants (Spearman’s rank correlation coefficient < 0.335). Even though BCAA concentrations were variable, their fermentation products were always greater post-RYGB. This observation was further supported by the predicted abundances of the genes that are involved in the BCAA (valine, leucine, and isoleucine) degradation and the synthesis of BCFA production pathways as shown in Fig. 4f. The predicted abundances of BCAA degradation genes were significantly greater after RYGB and in the RYGB-CS group in comparison to the pre-RYGB group. In summary, changes in the microbiome due to RYGB surgery seemed to enhance fecal amino acid metabolism, which may have contributed to weight loss by producing BCFA that are capable of signaling free fatty acid receptors48. The role of BCFAs on FFA receptor signaling warrants further investigation.
In addition to SCFAs and BCFAs, we analyzed a wide spectrum of other metabolites. Most of the fecal metabolites including sugars and amino acids, detected with 1H-NMR and GC-MS, were at greater abundance in the pre-RYGB group, and their concentrations dropped 12 months after surgery (Table 4). The fecal metabolite concentration profiles of RYGB-12_mo and RYGB-CS groups were similar, possibly due to the altered gastrointestinal tract environment after the surgery and similarities in participant diets. However, as shown in Table 4, besides isovalerate and isobutyrate, concentrations of xylose also increased after RYGB and were even higher than for NW controls. Greater abundance of fecal xylose after RYGB would seem to indicate that the participants adapted to more plant-based diets or that they lost some microbial hydrolytic capabilities to metabolize xylose. The (self-reported) participants’ fiber intake did not change significantly after the surgery (Table 1), although it was statistically lower in post-RYGB participants compared to NW participants (p = 0.032).
RYGB surgery decreased fecal bile acid concentrations
As RYGB is known to alter the bile acid metabolism49,50 and contribute to remission of type 2 diabetes and weight loss51, we quantified seven primary and 10 secondary bile acids in fecal samples from participants before and after RYGB surgery using liquid chromatography-mass spectrometry (LC-MS). Figure 5a, b and Table S2 show primary and secondary fecal bile acids and their conjugated forms measured at baseline, 6 months, and 12 months after RYGB. Fecal concentrations of primary bile acid—cholic acid (CA)—and its glycine- and taurine-conjugated forms (TCA and GCA) were significantly lower 6 months after the surgery (CA p = 0.022, TCA p = 0.001, GCA p = 0.002), and they remained at similar concentrations 12 months after surgery. Similarly, concentrations of glycine- and taurine-conjugated forms of chenodeoxycholic acid (CDCA), GCDCA and TCDCA, dropped significantly 6 months after the surgery. Concentrations of secondary bile acids, lithocholic acid (LCA), its glycine conjugated form, GLCA, and taurodeoxycholic acid (TDCA) significantly dropped 6 months after surgery as well (LCA p = 0.02, GLCA p = 0.001, and TDCA p = 0.003). Figure 6a illustrates the conjugation and transformation reactions of primary bile acids and the resulting secondary bile acids produced by gut microbiota52. Our findings show that primary and secondary bile acids were significantly diminished in feces after RYGB surgery.
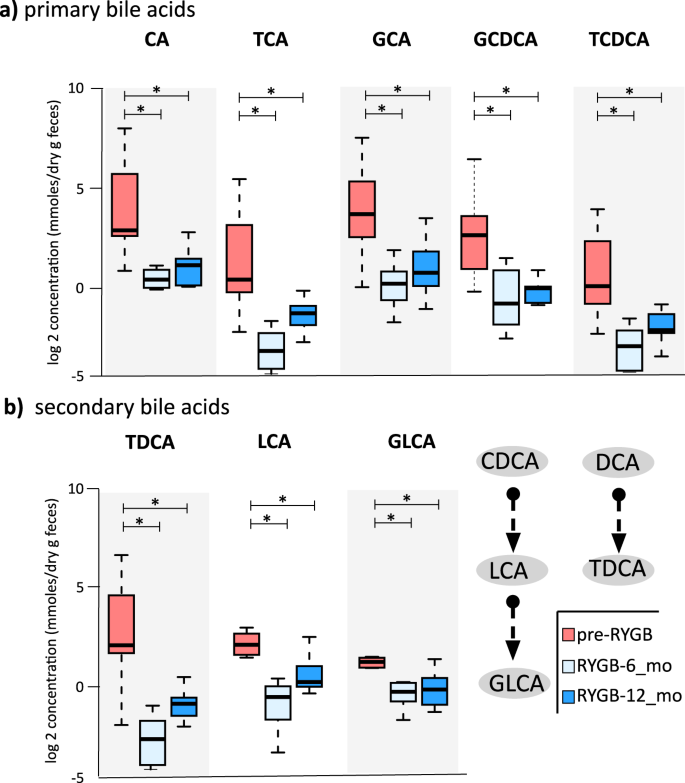
a Fecal primary bile acid (CA cholic acid, TCA taurodeoxycholic acids, GCA glycocholic acid, GCDCA glycochenodeoxycholic acid, TCDCA taurochenodeoxycholic acid) and b fecal secondary bile acids (TDCA taurodeoxycholic acid, LCA lithocholic acid, GLCA glycolithocholic acid) that were statistically different after RYGB surgery. * indicates statistical significance between pre-RYGB and RYGB-6 and RYGB-12_mo groups based on Wilcoxon signed-rank test and p values were corrected using Bonferroni method *p < 0.05. The box plots represent minimum, maximum, median, first quartile, and third quartile values.
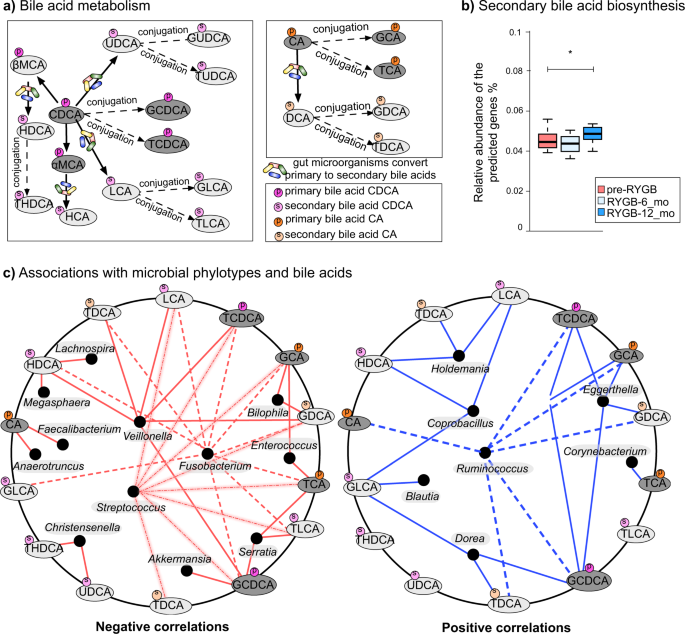
a Bile acid transformation reactions. Orange color = primary bile acids, green = primary conjugated bile acids, blue = secondary bile acids, pink = secondary conjugated bile acids. CA cholic acid, DCA deoxycholic acid, GDCA glycodeoxycholic acid, TDCA taurodeoxycholic acid, GCA glycocholic acid, TCA taurocholic acid, CDCA chenodeoxycholic acid, LCA lithocholic acid, HCA hyacholic acid, HDCA hyodeoxycholic acid, UDCA ursodeoxycholic acid, GUDCA glycoursodeoxycholic acid, TUDCA tauroursodeoxycholic acid, THDCA taurohydroxydeoxycholic acid, GLCA glycolithocholic acid, TLCA taurolithocholic acid, GCDCA glycochenodeoxycholic acid, TCDCA taurochenodeoxycholic acid, αMCA α-muricholic acid, βMCA β-muricholic acid. b Bile acid biosynthesis genes predicted from 16S rRNA gene abundances via PICRUSt. KO numbers that were used in the prediction: K01442, K00076, K23231, K22604, K22605, K22606, K22607, K15868, K15871, K15869, K15870, K15873, K15874, and K07007. The box plots represent minimum, maximum, median, first quartile, and third quartile values. c Fecal bile acid and microbiome co-occurrence network based on Spearman’s rho correlation coefficients.
In order to reveal microbial connections to bile acid metabolism, we used Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) software53 to predict secondary bile acid biosynthesis pathway from 16S rRNA gene abundances. PICRUSt prediction of secondary bile acid biosynthesis pathway was greater after RYGB; however, these are genomic predictions and not activity measurements (Fig. 6b). Table S2 summarizes the median concentrations of primary and secondary bile acids observed in NW and RYGB-CS groups in comparison to RYGB-12_mo and pre-RYGB groups. The concentrations measured in the RYGB-CS group were similar to the RYGB-12_mo group, which indicates that the response of surgical modification on bile acid metabolism was strong and reproducible, even if the baseline time points before the surgery are missing. Additionally, bile acid levels after RYGB groups were similar to NW participants (Table S2). Overall, our findings indicate that fecal concentrations of primary and secondary bile acids declined after RYGB surgery, and levels similar to those in NW individuals were maintained even years after the surgery. Fat and cholesterol intake are important factors in the production and secretion of bile acids52. As seen in Table 1, the participants did not reduce the fat percentage of their diets, although they consumed fewer calories after RYGB, which leads to lower absolute amounts of fat being consumed. Lower delivery of fat to the gastrointestinal tract might have played a role in the lower concentrations of fecal primary and secondary bile acids measured in this study.
Considering that gut microbiota can transform bile acids52,54 and concentrations of bile acids can affect gut microbiota composition52, we performed co-occurrence-network analysis between fecal genus–level microbial phylotypes and bile acids. As shown in Fig. 6c, phylotypes that were enriched after RYGB, including Fusobacterium, Veillonella, Enterococcus, Akkermansia, and Streptococcus negatively correlated with various bile acids such as TDCA, LCA, TCDCA, GCA, GDCA, TCA, and TLCA. Christensenella, a strongly heritable phylotype that was also associated with lean body type55, was the only genus-level phylotype that negatively correlated with the secondary bile acids THDCA and UDCA. Previously, UDCA treatment have been associated with weight gain in humans56. On the other hand, Ruminococcus, Coprobacillus, Holdemania, Eggerthella, and Dorea positively correlated with primary and secondary bile acids.
We performed the same analysis with mucosal genus-level phylotypes and bile acids (see Supplementary Fig. 5). We observed associations with minor taxa such as Methanobacterium and bile acids. Interestingly, Clostridium genus phylotypes negatively correlated with a number of bile acids. Additionally, UDCA, GDCA, and GUDCA were the bile acids that showed the greatest number of associations with mucosal phylotypes. Given that bile acids have been reported to modify the gut microbiome52, lower delivery of bile acids to the colon might have played a role on some of the microbiome compositional changes observed. Additionally, microbial bile acid metabolism can potentially have effects on host body weight and metabolism since it was previously shown that bile diversion to the small intestine can recapitulate some of metabolic benefits of the RYGB independently from the surgery57.
Previous studies in humans reported increased levels of circulating bile acids, especially secondary bile acids, after RYGB as measured in blood plasma25,51,58. A recent study characterizing bile acids in the fecal samples in women after RYGB showed decreased concentrations of many bile acids59; hence, our results support findings from that study. In rats, RYGB has also been shown to increase plasma bile acid concentrations and the secretion of weight-loss-associated hormones Peptide YY and Glucagon Like Peptide-1 (ref. 49). However, a recent study on rats demonstrated that the bile acid profiles in the intestines did not change after RYGB even though microbial profiles were significantly altered60. One difference among the reported human studies and ours is that our measurements were in fecal samples, whereas the others analyzed serum samples; hence, the measurements are not directly comparable. Bile acid quantification is often done in serum samples, which might reflect more physiologically relevant concentrations. However, our findings in fecal samples may lead to more profound understanding of microbial metabolism of bile acids in the gut. Further studies on the impact of microbial metabolism and gut levels of bile acids on host health are warranted.
We demonstrated the impact of RYGB surgery on the gut microbiome, metabolome, and bile acid metabolism of humans studied prospectively and retrospectively. We document that changes in the human gut microbiome after RYGB in the luminal and mucosal space. The mucosal space is a critically important site for host–microbe interactions. Changes in the fecal metabolome mirrored changes in the fecal and mucosal microbiome structure, suggesting that the profile of microbial metabolism changed as a result of major physiological, environmental, and nutritional alterations affecting the gut after RYGB surgery. The delivery of bile acids to the colon diminished after surgery, potentially contributing to the altered microbiome and metabolome profiles. As a small sample size is a limitation of our study, studies with greater sample size are needed to validate our findings. Finally, results from a longitudinal cohort were consistent with observations from cross-sectional studies after RYGB surgery, supporting a dominant and persistent impact of RYGB on the intestinal microbiome.
Source: Ecology - nature.com


