In natural systems, the black shale oxidation process is usually involved in multiple factors, such as chemical and biological mechanisms29,36. Previous studies have shown an effective pyrite oxidation ability presented in biological system such as A. ferrooxidans22, but few investigations have discussed its direct relationship to black shale oxidation. Considering the oxidation process initiated at the rock surface, we assumed a correlation between rock micro-surface characteristics alteration and oxidation degree, especially at the initial stage. Therefore, in this study, a comparative experiment is designed and carried out on black shale slices, the pyrite oxidation rate is employed to the index for black shale oxidation degree, aim to reveal a core difference in chemical and biological oxidizing black shale at the initial stage.
Based on the micro-surface morphologies result, it can be seen that both the acid solution and A. ferrooxidans are effective against pyrite oxidation. In G1 and G3, the pyrite assemblage almost disappeared after reactions, whereas it remains on the rocks surface in G1 (Fig. 1). The XRD results indicate that the most reduction of pyrite content is presented in G3. General speaking, the pyrite oxidation in black shale is accompanied by the microstructure decomposition associated with the damage of shale matrix cement11,37. However, this correlation seemed only occurred in abiolotic systems. The more robust pyrite oxidation led to the more clay minerals and irregular flocculent structures formed in G2 compared with that in G1. These results are consistent with previous findings14 that the acidic environment is facilitated to black shale oxidation. Whereas in the biolotic system, it is difficult to assess the micro-structure destruction, because of large amounts of jarosite covering on the rocks surface. Based on a field investigation, Liao et al.14 also reported a considerable amount of jarosite precipitation occurred on weathered black shale surface. Therefore, the relationship between micro-surface morphologies and the pyrite oxidation rate is different in abiolotic and biolotic systems.
Black shale is low in porosity and permeability, the oxidation process is controlled by “active” porosity which enables the fluid flow through into interior subsurface11. In the initial oxidation stage, we assume that the alterations of porosity and permeability would be limited to the micro-surface. However, the classic porosity measurement methods, such as nitrogen adsorption and high-pressure mercury intrusion tests seem unsuitable to the surface porosity measurement in this study. For example, previous studies by utilizing nitrogen absorption analysis documented the overall porosity distribution of black shale is major micro- and meso-pores with the pore diameter less than 50 nm, where fluid is difficult to flow through and led to further reaction38, but they are unable to distinguish the contribution of surface porosity, which may significantly different to the internal porosity. By using a novel fluorescent staining method, the fluid distribution on the rock surface can be illustrated, since this fluorescent dye can be detected only when it occurred in aqueous. We suppose that rinsing with neutral PBS can remove the fluid not contained in the rocks surface pores, therefore, the fluid distribution images can be employed to reveal the porosity on the rocks surface. Another feature of this dye is the color dependence on aqueous pH, which is yellow in acidic environment but turned to green when approach to neutral solution. Thus the MpH can also measure the pH of fluid distributed in the surface pores.
As demonstrated in Fig. 2, the fluorescent density is low in the original samples, indicating a low porosity of the rock surface. Although the total square of fluorescent density does not significantly change after reactions, the MpH is different to the original images and varies among groups. The low MpH areas increase more in G2 than that in G1, indicating more acid solution contained in the rocks surface pores in G2. These results agree with the alterations of micro-surface morphologies, the more pyrite oxidation results in more increase of acid solution permeability. But this interpretation can not apply to G3, in regard of a similar aqueous pH and pyrite oxidation rate presents in G2 and G3. The MpH does not significantly change in G3 by comparing with the original state, it probably since the surface jarosite coverage blocks the acid solution permeability. In addition, the carbonate also exists in the samples, its buffer ability may effect the MpH values, but considering the same experimental condition in all samples, this influence could be similar in three groups. Nevertheless, the distinct MpH among groups suggests the alternation of micro-surface porosity and permeability is different in biolotic and abiolotic systems.
Laboratory studies showed that the acid erosion could promote original matter oxidation and field investigations exhibited the organic matters nearly removed from the regolith2,39, these results indicated that the organic matters were depleted during black shale weathering process. Although a biological mechanism for the oxidation of pyrite by A. ferrooxidans has been intensively investigated21,22,23,24,25,26,27, very little work on its roles in organic matters oxidation process. Through EDX line scans measurement, we analysis the relative proportion of elements on the rocks micro-surface with aspect to a 2μm depth, to evaluate the elemental alteration on the rock micro-surface. As shown in Fig. 3, the relative proportion of elemental C, Fe, Mg, Al, S and K does not significantly change in G1, but markedly decreases in G2 after reaction. Although the overall content of minerals shows a small variation in G1 and G2, a more obvious minerals dissolution may occur on the rocks micro-surface in G2 in accordance with a more significant micro-structure destruction. Whereas in G3, which has a similar aqueous pH values with G2, the elemental C concentration increases after reactions. It may due to the attachment of A. ferrooxidans cell bodies on the rocks micro-surface, as numerous cell bodies are observed on the rocks micro surface in G3 (Fig. 1). Likewise, with the humidity climate of Pennsylvania, Jin et al.11 observed an addition of carbonate profile in the Rose Hill that may cause by bio-turbulence. Despite the EDX analysis just show the relative elemental C alterations, it can not equal to the total organic carbon (TOC) quantity, but for the micro-surface elemental C concentration, it shows depletion pattern in abiolotic system but addition pattern in biolotic system.
Previous literature documented a more effective oxidation ability presented in A. ferrooxidans compared with acid water alone, however, most of these approaches were inferred from pure pyrite22. If it assumed that the biological oxidation of black shale is much higher or even same the magnitude of the chemical effect, the overall rate of the biological oxidation processes should be re-estimated. However, based on field observations, the biological effect is usually limit to the rock surface29. The underlying mechanisms may relate to the different micro-surface alternations in chemical and biological oxidizing black shale, especially at the initial stage. In the proposed reactions at the initial oxidation stage, the pyrite embedded in black shale exhibited differential oxidation behavior in chemical and biological systems. In this regard, only effects of the first 7days are investigated in this study. Despite previous studies demonstrated a more obvious difference occurred in longer term experiments22, but which is not the focus of present study.
The pyrite oxidation rate is utilized as an index for black shale oxidation, which is higher in biolotic system at the initial 4 days. As shown in Table 1 and Fig. 4, the pyrite content decreases and the aqeoues ORP increases the most in G3, accompanied with the highest Fe3+ ions concentrations, these results are consistent with findings from pure pyrite22. However, the ORP and Fe3+ ions concentrations decreases from the 5th day, indicating a deceleration of pyrite oxidation rate. Based on the results of micro-surface charateristics, this decrease is primarily attributed to the jarosite formation and covering on the rock surface.
The jarosite formation is a common problem of A. ferrooxidans bio-leaching engineering applications21, but is seldom reported to black shale biological oxidation mechanism. It is clear from above results that the jarosite formation is A. ferrooxidans dependence, the underlying mechanism may associate with following reasons. Initially, A. ferrooxidans cell surfaces served as nuclei for crystal growth of jarosite, subsequent cell metabolism will increase the amino acids, such as glycine and proline, which can significantly impact the morphology, yield and crystallinity of jarosite40,41. With the pyrite oxidation process ongoing, the ferric iron and sulfur concentrations increase, resulting in acceleration of jarosite formation35,40. Finally, the formed jarosite prefers to deposit on the rocks surface rather than dissolve in the biolotic aqueous systems40. Electro kinetic investigations have proposed that the changes in surface charge, such as a function of pH and ferric iron was the basis of pyrite oxidation21,35, however, the surface jarosite coating might passivate against this reaction. Additionally, the accumulation of carbonates on the rocks surfaces could also make a contribution to inhibit further reactions.
Therefore, two phases of reactions may involve in A. ferrooxidans oxidizing black shale at the initial stage. Firstly, A. ferrooxidans cells primarily attach on the rocks surface and then oxidize the ferrous iron into ferric iron, as well as the sulfur, in this phase, the pyrite oxidation rate is significantly prompted by A. ferrooxidans which is also recognized as “pyrite oxidized phase” (Fig. 5a). With the accumulation of ferric iron and sulfur, jarosite is formed and precipitated on the rocks surface, resulting in alteration of surface characteristics and inhibition of further reactions. Thus, this phase is so-called the “jarosite formation phase” (Fig. 5b)
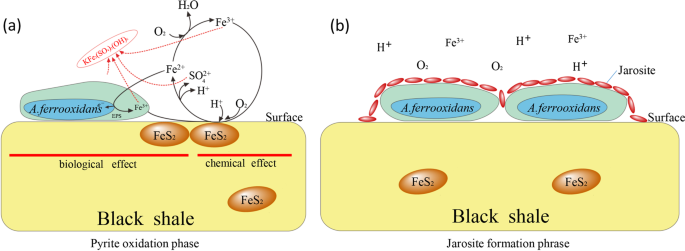
The schematic diagram of A. ferrooxidans biological weathering on black shale in pyrite oxidation phase (a) and jarosite formation phase (b). EPS, extracellular polymeric substances.
It is notably that although a link between micro surface characteristics and black shale oxidation degree at the initial stage is founded in acid solution and A. ferrooxidans, it is still difficult to clearly interpret the difference in chemical and biological effects on black shale oxidation. For example, the pH in chemical system is at 2.50, which is much acid than bedrocks or regolith soils in natural black shale13,14. Furthermore, there are multiple sources of microorganisms presented in local environment except for A. ferrooxidans42, the contributions of other microorganisms should not be ignored which need further investigation. Finally, given there are multiple minerals contained in black shale, the future work will be focused on their roles in the overall oxidation process of black shale.
Source: Ecology - nature.com


