Opposite modulation of TbGMPR activity by purine nucleotides
Enzymes harboring a CBS domain usually change their activities in the presence of purine nucleotides10,11,12; therefore, we sought to examine whether purine nucleotides modify the activity of TbGMPR. In the presence of GTP, the initial velocity of TbGMPR was upregulated in a concentration-dependent manner (Fig. 1a), and the EC50 value was estimated to be 4.8 µM. In contrast, TbGMPR exhibited a decrease in its initial velocity in the presence of ATP with an EC50 value of 160 µM (Fig. 1b). The effect of each triphosphate nucleotide was maintained when added as a magnesium complex, i.e. Mg-GTP or Mg-ATP (Fig. 1a, b). These results indicate that TbGMPR is positively and negatively regulated by GTP and ATP, respectively, with and without magnesium ions. Kinetic analysis demonstrated that the initial reaction velocity of TbGMPR without ligand nucleotides showed a sigmoidal curve when plotted against the concentrations of GMP, and the plots were well-fitted to the Hill equation (Fig. 1c, open circles). The kinetic parameters K0.5 and kcat values for GMP were determined as 184 ± 3 µM and 16.7 ± 0.15 min−1, respectively (mean ± s.d.; Table 1). The Hill constant (nHill) was calculated to be 3.04 ± 0.12, meaning that GMP induced a positive cooperativity effect on TbGMPR. A similar sigmoidal curve was observed using recombinant TbGMPR prepared via an affinity purification with glutathione-S-transferase (GST) tag and subsequent tag-removal (Supplementary Fig. 1). These results indicate that GMP induces a positive cooperative effect on TbGMPR, both with and without a terminal tag.
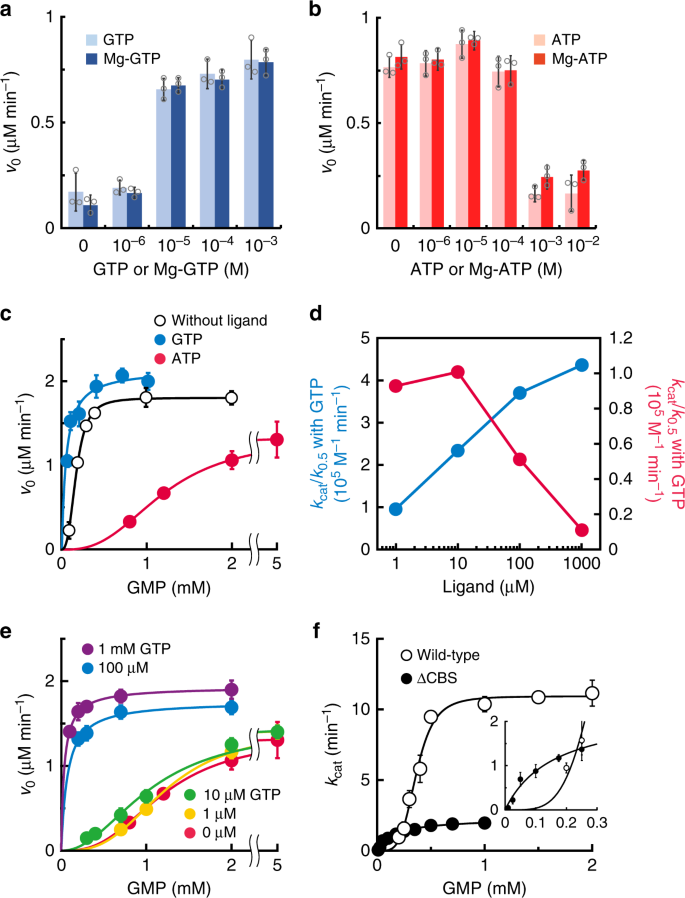
a, b The initial velocities of TbGMPR in the presence of GTP (a) or ATP (b) at a fixed concentration of GMP and NADPH. Each trinucleotide was used as a premix with (solid bars) or without (shaded bars) equivalent amount of magnesium ions. c The initial velocities of TbGMPR were plotted against the concentrations of GMP in the absence (open circles) or presence of 1 mM GTP (blue) or ATP (red) at a fixed concentration of NADPH. Data were fitted to the Hill equation, as described in the “Methods”. d The catalytic efficiency (kcat/K0.5) of TbGMPR in the presence of various concentrations of GTP (blue) or ATP (red) ligand alone. e The initial velocities of TbGMPR in the absence (red) or presence of 1 (orange), 10 (green), 100 µM (blue), or 1 mM GTP (magenta) under fixed concentration of ATP at 1 mM. Note that the kinetics with GTP at 100 µM and 1 mM showed a Michaelis–Menten-like profile. f The kcat values of TbGMPR (open circles) and TbGMPR∆CBS (closed circles) were plotted against the concentrations of GMP. The inset shows the data at low concentrations of GMP. Data were obtained from three independent experiments (n = 3). Each data point represents a mean ± s.d. in error bars. Source data are provided as a Source Data file.
The addition of GTP to the reaction mixture enhanced TbGMPR activity by decreasing the K0.5 accompanied with the increase of the kcat value in a concentration-dependent manner (Table 1, Fig. 1c and Supplementary Fig. 2). The nHill value in the presence of 1 mM GTP was 1.00 ± 0.34. In contrast, ATP showed an inhibitory effect on TbGMPR activity by lowering the substrate–enzyme affinity as indicated by the K0.5 value of 1200 ± 12 µM, while the kcat and nHill values were relatively unchanged compared to those obtained in the absence of ligands (Table 1, Fig. 1c and Supplementary Fig. 2). The increase in the K0.5 value was observed in an ATP concentration-dependent manner (Supplementary Fig. 2a). The opposed effects of GTP and ATP on TbGMPR activity were clearly observed when the values of catalytic efficiency (kcat/K0.5) were plotted against each ligand concentration. Although the kcat/K0.5 value of TbGMPR in the absence of the ligands was determined as 0.913 × 105 M−1 min−1, it was increased by the addition of 10 µM GTP, and finally showed 4.36 × 105 M−1 min−1 with 1 mM GTP (Fig. 1d and Table 1). In contrary, the kcat/K0.5 value was decreased by the addition of ATP and reached to 0.111 × 105 M−1 min−1 with 1 mM ATP (Fig. 1d and Table 1). These results indicate that guanine and adenine nucleotides are the allosteric regulators responsible for the positive and negative regulation of TbGMPR activity, respectively, by altering the affinity of the substrate GMP for the reaction center.
To investigate the regulation of TbGMPR activity by purine nucleotides under the physiological conditions, we examined whether GTP can activate the ATP-inactivated enzyme by measuring the activities in the presence of both 1 mM ATP and various concentrations of GTP. At the concentrations of 10 µM or less, GTP was ineffective on ATP-inactivated enzyme, however, 100 µM GTP was sufficient to revoke the inhibitory effect of ATP (Fig. 1e). The nHill value in the latter condition was calculated to be 1.03 ± 0.68; this was very close to the value in the presence of GTP alone rather than that without the ligands. Inversely, the addition of 3 mM ATP inhibited the 100 µM GTP-activated TbGMPR, whereas 1 mM ATP showed only a moderate effect (Supplementary Fig. 3a). ATP was less effective to 1 mM GTP-activated TbGMPR (Supplementary Fig. 3b). These data indicate that TbGMPR is reversibly regulated between the activated and inhibited state by binding of GTP and ATP, respectively, and this state transition is dependent to the concentration ratio of these nucleotide ligands.
Allosteric binding of purine nucleotides to CBS domain
We investigated the binding of ligand nucleotides to the CBS domain of TbGMPR by fluorescence quenching of the tryptophan residues. TbGMPR has two tryptophan residues that are located in its CBS domain (Trp115 and Trp120). To avoid interference between the two tryptophan residues, we replaced Trp115 with arginine (TbGMPR W115R). The enzymatic properties of TbGMPR W115R were almost identical to those of the wild-type enzyme (Supplementary Fig. 4). In the presence of ligand nucleotides, TbGMPR W115R clearly showed fluorescence quenching of Trp120 in a ligand concentration-dependent manner (Fig. 2, upper panels). We also observed blue-shifting of the emission spectra of the protein with either GTP or GMP, but not with ATP, in a nucleotide concentration-dependent manner. These data suggest that the local environment around Trp120 is rendered more hydrophobic in the presence of guanine nucleotides. When plotting the peak fluorescence intensities against the ratios of ligand to enzyme concentrations, the data for each ligand except GMP showed a curve-fitting to the Hill equation (Fig. 2, lower panels), and the dissociation constants for GTP and ATP were calculated as 6.75 ± 0.58 and 194 ± 45 µM, respectively. Supplementation of magnesium ions showed little or no effect on the spectrum of TbGMPR with GTP or ATP (Supplementary Fig. 5). These results indicate that the purine nucleotides used here bind close to Trp120 in the CBS domain of TbGMPR, and the bindings are independent of magnesium ions.
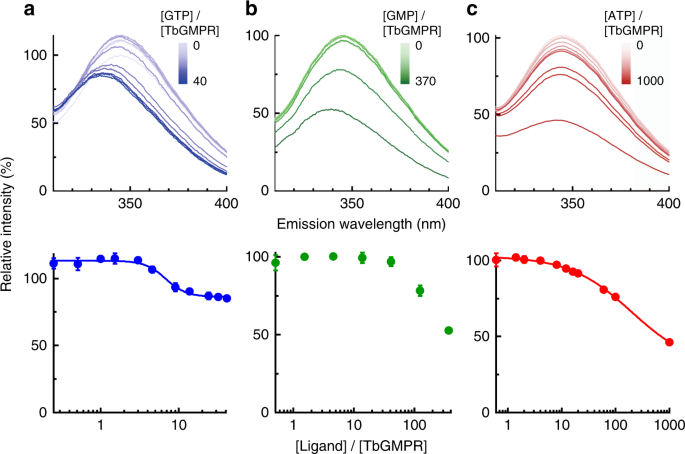
a–c (Upper panels) The fluorescence emission spectra of Trp120 in TbGMPR W115R. GTP (a), GMP (b), and ATP (c) were used as ligands. a–c (Lower panels) The peak value of each plot was re-plotted against the ratio of the ligand to protein concentration shown in logarithmic scale. Plots were fitted to the Hill equation. Data were obtained from three independent experiments (n = 3). Each data point represents a mean ± s.d. in error bars. Source data are provided as a Source Data file.
Substrate-induced positive cooperativity via CBS domain
We generated a mutant version of the TbGMPR protein lacking the CBS domain (TbGMPR∆CBS) and used this altered protein to elucidate the involvement of the CBS domain in purine nucleotide-dependent regulation of TbGMPR. TbGMPR∆CBS showed a marked decrease in overall activity, exhibiting a fivefold decrease in kcat value compared to that of the wild-type (Fig. 1f and Table 1). However, the K0.5 values of the wild-type and TbGMPR∆CBS were comparable, and the nHill for TbGMPR∆CBS with GMP was 1.00 ± 0.14. These results indicate that deletion of the CBS domain eliminates the positive cooperative effect of GMP on TbGMPR activity while having little effect on the affinity for substrate binding to the catalytic center. In addition, the addition of purine nucleotides other than GMP had no effect on the activity of TbGMPR∆CBS (Supplementary Fig. 6).
TbGMPR architecture
To obtain direct evidence for the allosteric binding of purine nucleotides to the CBS domain of TbGMPR, we attempted crystallization, with or without purine nucleotides, of the inactive mutant TbGMPR C318A, in which the catalytic residue Cys3186 was replaced by an alanine. We obtained the crystal without ligand (C318A apo) and successfully determined the structure, at 2.80-Å resolution, of a GMPR harboring a CBS domain (Supplementary Table 1). A monomer of C318A apo comprised two domains: the catalytic domain (Ser2–Phe97 and Arg226–Gly484) and the CBS domain (Leu98–Ser225). The former displayed a typical TIM-barrel fold with accessory α-helices and antiparallel β-sheets, whereas the latter had a tandem repeat of α–β–β–α folds characteristic to all CBS domains known to date. PISA data analysis13 revealed that a tetramer (referring to as tetramer 1 or 2) was formed from four subunits (subunits A–D or A′–D′) related by a fourfold axis, and that the two tetramers were related by a twofold axis perpendicular to the fourfold axis to constitute an octamer (Fig. 3a). The interactions between the adjacent subunits in the tetramer involved a large number of hydrogen bonds and hydrophobic interactions, whereas the formation of the octamer was stabilized by a hydrogen bond and 14 C–C contacts between the CBS domains of subunit A in tetramer 1 and subunit A′ in tetramer 2, and equivalent interactions between subunit B and B′, C and C′, and D and D′ (Supplementary Tables 2 and 3). However, no interaction was observed between the catalytic domains, indicating that the CBS domain is essential for the formation of the TbGMPR octamer.
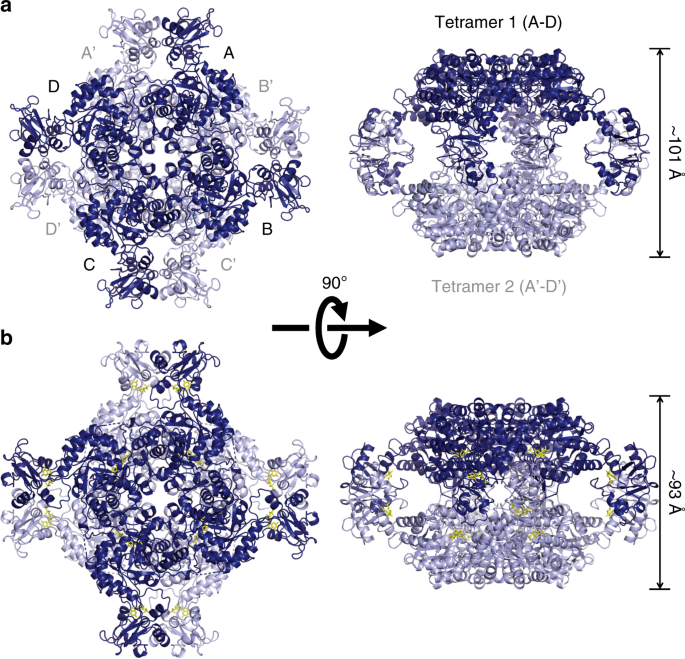
Cartoon representation of the octameric structures of C318A apo (relaxed form, a) and C318A/GMP (twisted form, b), each of which are composed of two tetramers. Tetramer 1 (subunits A–D) and tetramer 2 (subunits A′–D′) are colored in light and dark blue, respectively. GMP molecules are shown as yellow stick representations. Note that C318A/GMP (b; right panel) is compressed compared to C318A apo (a; right panel).
We also determined the structure of TbGMPR C318A with substrate GMP (C318A/GMP) at 1.90-Å resolution and found that C318A/GMP adopted an octameric structure as observed in C318A apo (Fig. 3b and Supplementary Tables 1–3). However, two tetramers in the C318A/GMP octamer were arranged in a twisted position around the fourfold axis compared to C318A apo, and the overall structure of the C318A/GMP octamer was compressed along the fourfold axis. Thus, it appears that GMP induces the transformation of TbGMPR from a “relaxed” (C318A apo) to a “twisted” form (C318A/GMP). Two GMP molecules were observed in each subunit of C318A/GMP; one was found in the active site, whereas the other was bound to the cleft between the catalytic and CBS domains (Fig. 4a). The interactions between the active site and the substrate GMP molecule involved 12 hydrogen bonds and 7 C–C contacts (Supplementary Fig. 7 and Supplementary Table 4). The interactions between the base moiety of GMP and three amino acid residues (Met401, Ala402, and Glu428) in the catalytic domain appeared to stabilize the α-helix and a portion of the following loop structure that were disordered in C318A apo (Fig. 4b, c). The conformation induced upon substrate binding is considered to have a key role in GMPR activity.
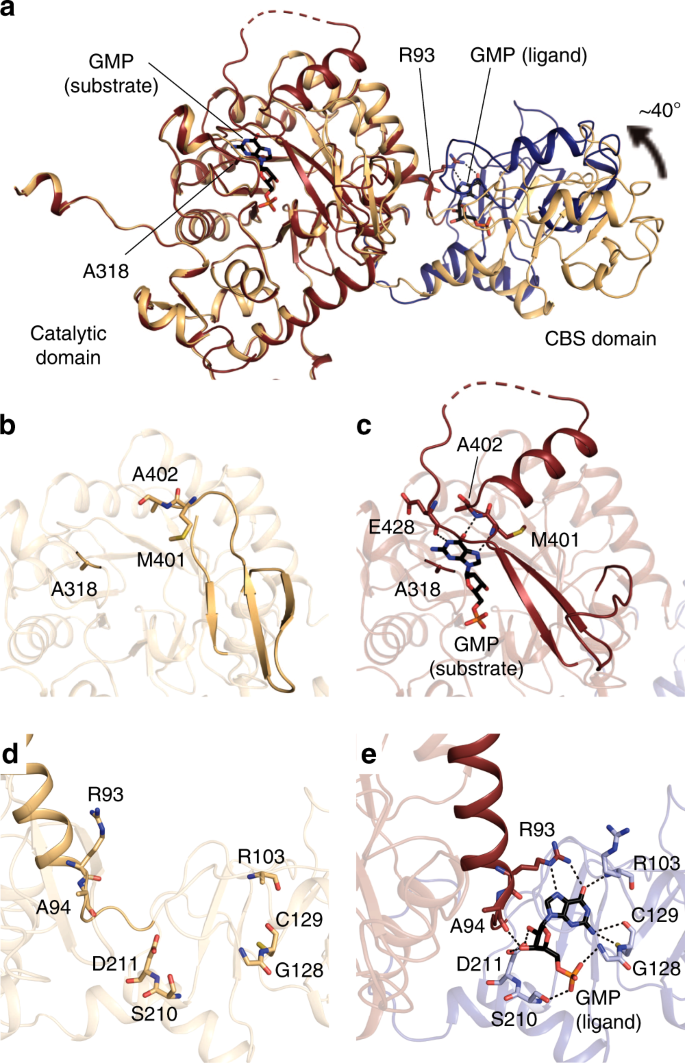
a Cartoon representation of C318A/GMP and C318A apo subunits with the catalytic domains superimposed. The catalytic and CBS domains of C318A/GMP are colored in red and blue, respectively, and the overall structure of C318A apo is shown in orange. b–e Enlarged views of the active site (b, c) and the allosteric regulatory site (d, e) of C318A apo (b, d) and C318A/GMP (c, e). The hydrogen bonds are depicted as dashed lines. Note that Arg93 on the α-helix of the catalytic domain forms hydrogen bonds with the GMP at the allosteric regulatory site.
The ligand GMP molecule at the allosteric regulatory site formed 10 hydrogen bonds to and exhibited 13 hydrophobic interactions with the amino acid residues in the CBS domain; at the same time, the O6 and N7 atoms in the GMP molecule interacted with Arg93 in the catalytic domain via hydrogen bonds (Fig. 4e and Supplementary Table 5). The structures of the isolated catalytic and CBS domains in C318A/GMP could be well-superposed with the respective domains in C318A apo (Supplementary Fig. 8). However, the relative orientation of these domains was obviously different between C318A/GMP and C318A apo: the CBS domain is rotated ~40° between these two structures (Fig. 4a), a change that may be induced by an interaction between the base moiety of GMP and Arg93 (Fig. 4d, e). This hinge motion is inferred to contribute to the transformation from the relaxed to the twisted octamer. We further determined the structure of the wild-type TbGMPR complexed with GTP (TbGMPR/GTP) at 2.50-Å resolution (Supplementary Fig. 9 and Supplementary Table 1). In this structure, single GTP molecules were found only at the allosteric regulatory site on each subunit of TbGMPR/GTP, indicating that GTP acts only as a ligand. The overall structure was almost identical to that of C318A/GMP (RMSD = 0.66 Å for Cα atoms) (Supplementary Fig. 9b). The hinge motion in each subunit and the twisted conformation of the octamer upon allosteric binding of the ligand were also observed in TbGMPR/GTP (Supplementary Fig. 9b, c).
Regulation of oligomeric state by ligand nucleotide binding
It is important to study the structure of the protein not only in crystal but also in solution to understand its native structure in a physiological environment. We employed SEC-SAXS analysis to evaluate the oligomeric state and the conformation of TbGMPR with and without ligands (Fig. 5a). In the absence of ligands, the scattering curve showed the local minimum at a q value of approximately 0.07 Å−1; however, the addition of GMP or GTP shifted the valley toward large q values. Meanwhile, the addition of ATP yielded a curve with no valley, a profile that was clearly distinct from those of the other curves. Kratky plots of TbGMPR with and without ligands exhibited a peak at Guinier–Kratky point in all measurements, but except for ATP, indicating that TbGMPR substantially adopts the globular conformation (Fig. 5b). However, the plot of TbGMPR in the presence of ATP showed a peak-shift from Guinier–Kratky point, and had no valley and diverged tailing at higher q × Rg region. These observations signify that ATP-binding results in an increase of the flexible structure in TbGMPR, leading the subunit conformation into a less compact form. The calculated radii of gyrations (Rg), maximum dimensions (Dmax), and molecular masses are summarized in Tables 2 and 3. Porod volumes and I(0) values are shown in Supplementary Table 6. We also carried out SEC-SAXS analysis for TbGMPR∆CBS, so as to investigate the effect on the oligomer formation of deletion of the CBS domain (Table 3 and Supplementary Figs. 10 and 11). The molecular mass of TbGMPR∆CBS calculated from the scattering curve was 137 kDa, which was comparable to the theoretical mass of its tetramer (TbGMPR∆CBS monomer, 39.2 kDa; Supplementary Fig. 12). These results indicate that the CBS domain in TbGMPR is essential for the octamerization of TbGMPR, and therefore, deletion of the CBS domain presumably prevents the interaction between adjacent tetramers.
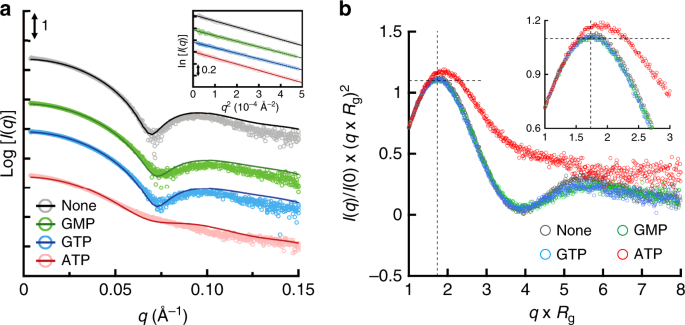
a SAXS intensities for TbGMPR in the absence (black) or presence of GMP (green), GTP (blue), or ATP (red) were plotted against the scattering vector q defined as 4πsinθ/λ. The scattering intensity I(q) was represented in absolute intensity. The fitting curves were calculated by OLIGOMER. The inset shows the Guinier plots of each curve. b Dimensionless Kratky plots of TbGMPR in the presence of purine nucleotides. The plots were calculated for TbGMPR in the absence (gray) or presence of GMP (green), GTP (blue), or ATP (red). Cross-hair marks the Guinier–Kratky point (1.732, 1.1), the main peak position for globular particles. The inset shows the enlarged view of the peak position. Data were obtained from three independent experiments (n = 3). Source data are provided as a Source Data file.
In order to evaluate the oligomeric states and the conformations of TbGMPR in solution with or without ligands, we applied the program OLIGOMER to obtain fitting curves for the experimental SAXS data based on a linear combination of the theoretical scattering curves of three conformations. Two of the three conformations were the crystal structures of C318A apo and C318A/GMP that represent the relaxed and the twisted octamers, respectively; the third conformation was a tetramer structure generated by dividing the C318A/GMP octamer in half. The OLIGOMER analysis demonstrated that TbGMPR without purine nucleotides was found only as an octamer, though the proportion of the relaxed and twisted forms were estimated as 53.2% and 46.8%, respectively, whereas TbGMPR in the presence of guanine nucleotides was found almost completely as the twisted form (Table 3). In the presence of ATP, however, 60.8% of TbGMPR was found as the tetramer, with the rest of the protein present in the relaxed (28.0%) and twisted (11.2%) octamer conformations. These results suggest that changes in the oligomeric state contribute to the allosteric regulation of TbGMPR by purine nucleotides.
Source: Ecology - nature.com


