A recent study linked Z10-C33 to Varroa-infested, DWV-infected, and hygiene-targeted honey bee brood50. Given the structural similarity of Z10-C33 to Z6-C15, previously linked to hygienic removal49, we decided to investigate the effectiveness of Z10-C33 and Z6-C15 in triggering hygienic behavior. For this purpose, Varroa-Sensitive Hygienic (VSH), Minnesota Hygienic (HYG), and unselected control (UNS) honey bee colonies were established at the University of North Carolina at Greensboro apiary in the Spring of 2017. The VSH queen was sourced from the USDA-ARS Honey Bee Breeding Laboratory in Baton Rouge, where queens are selected based on suppression of mite reproduction. The HYG queen was sourced from the well-established breeder Jeff Hull (Minnesota) and was selected based on removal of>95% freeze-killed brood. The UNS queen was sourced from Triad Bee Supply which obtains their queens from Gardner Apiaries in Baxley, Georgia. For each experimental hydrocarbon, a control hydrocarbon of similar size and structure, but not known to be a component of the honey bee’s cuticular hydrocarbons, was also tested. Hydrocarbons (Z10-C33, Z16-C32, Z6-C15, and Z7-C15) were synthesized by Z-selective Wittig reactions between the appropriate aldehydes and phosphonium salts, or by Z-selective olefin metathesis reactions. Crude products were purified in two steps, by flash vacuum chromatography on silica gel, eluting with hexanes, followed by recrystallization from acetone at ~4 °C for longer chain compounds, or ~−20 °C for shorter chain compounds. Synthesis is described in further detail, below. Dilutions in hexane of 0.1%, 0.3%, and 1.0% (wt/vol) were prepared for each hydrocarbon. The lowest dilution is equivalent to that previously reported49, and higher dilutions were chosen to approximate dose effects on a logarithmic scale. All sample collections and analyses were conducted at the University of North Carolina at Greensboro.
Synthesis of alkenes tested in bioassays
Synthesis of Z6-C15
A solution of 1-decyne (5.52 g, 40 mmol) and ~50 mg triphenylmethane indicator in dry THF under Ar was cooled to ~−15 °C in an ice/acetone bath, and butyllithium (2.6 M in hexanes) was added dropwise until the solution turned pink. An additional 15.4 ml of butyllithium solution (40 mmol) was then added over 30 min, and the resulting solution was warmed to room temperature and stirred 1 h. Powdered NaI (0.6 g, 4 mmol) was then added, followed by dropwise addition of bromopentane (3.93 g, 26 mmol). The mixture was heated to reflux and stirred 22 h, then cooled and quenched with 1 M aqueous NH4Cl solution, and extracted with hexane. The hexane layer was washed with saturated NaHCO3 and brine, then dried and concentrated. The residue was purified by Kugelrohr distillation, taking a forerun of the excess 1-decyne (oven temp <40 °C, 0.05 mm Hg), then changing the collection bulb and collecting the desired product (2.8 g, bp~60 °C, 0.05 mm Hg).
The distilled product was flushed through a plug of silica gel with hexane and into a 200 ml round-bottomed flask with a magnetic stir bar. Lindlar catalyst (150 mg) and quinolone (1.5 ml) were added, and the flask was sealed and flushed with nitrogen, then hydrogen. With the sealed flask attached to a gas burette filled with hydrogen, stirring was then started, resulting in uptake of ~310 ml of hydrogen, at which point uptake virtually ceased. The flask was flushed with nitrogen, and the mixture was filtered through a plug of celite, rinsing well with hexane. The resulting solution was washed twice with 1 M HCl, then dried and concentrated. The residue was flushed through a pad of silica gel with hexane, then Kugelrohr distilled (2.82 g, bp~60 °C, 0.05 mm Hg). Because the resulting product was contaminated with about 4% of the alkyne starting material, a portion (1.2 g) was repurified by vacuum flash chromatography on silica gel in a 60 ml Buchner funnel. The silica was prewetted with hexane, then the impure alkene was loaded as a hexane solution, and the column was eluted with 5 ×30 ml hexane. Z6-C15 eluted cleanly in fractions 1 and 2, with the alkyne eluting in fractions 4 and 5. EI Mass spectrum (m/z, abundance): 210 (53, M+), 182 (3), 168 (1), 154 (2), 140 (4), 125 (15), 111 (41), 97 (79), 84 (42), 83 (90), 70 (69), 69 (100), 57 (59), 56 (59), 55 (91), 43 (42), 41 (56).
Synthesis of Z7-C15
Z7-C15 was made in analogous fashion from 1-octyne and 1-iodoheptane. EI Mass spectrum (m/z, abundance): 210 (41), 182 (3), 168 (1), 154 (2), 140 (4), 125 (13), 111 (38), 97 (76), 84 (43), 83 (89), 70 (69), 69 (100), 57 (59), 56 (63), 55 (94), 43 (63), 41 (64).
Synthesis of Z16-C32
1-Heptadecene (2.38 g, 10 mmol; GFS Chemicals, Powell OH) was flushed through a plug of silica gel with hexane, then concentrated and transferred to a dry 3-neck flask. The flask was flushed thoroughly with Ar while stirring and warming to 45 °C. Grubbs catalyst C675 (190 mg, 0.25 mmol; gift from Materia Inc., Pasadena CA) was added in one portion, and the mixture was stirred 3 h at 45 °C under a slow flush of nitrogen to remove the ethylene formed. The mixture was then cooled, diluted with hexane, and flushed through a plug of silica gel with hexane. The resulting semicrystalline residue was Kugelrohr distilled to 105 °C (0.05 mm Hg) to remove unreacted 1-heptadecene, and the residue was recrystallized from hexane at −20 °C. The resulting white crystals (1.04 g) were 99.6% chemically pure by GC. The Z/E ratio (>99% Z) was checked by epoxidation of a sample with meta-chloroperbenzoic acid in methylene chloride, followed by GC-MS; the trans-epoxide eluted before and was completed separated from the cis-epoxide. EI Mass spectrum (m/z, abundance): 448 (20), 420 (2), 376 (1), 362 (1), 334 (1), 320 (1), 306 (2), 292 (2), 278 (2), 264 (2), 250 (2), 236 (3), 222 (3), 210 (3), 196 (4), 181 (5), 167 (7), 153 (10), 139 (16), 125 (34), 111 (61), 97 (100), 85 (37), 83 (82), 71 (50), 69 (56), 57 (74), 55 (52), 43 (54), 41 (25).
Synthesis of Z10-C33
Triflic anhydride (2.2. ml, 12 mmol) was added dropwise to a slurry of docosanol (3.26 g, 10 mmol), pyridine (0.8 ml, 10 mmol), and ~50 mg dimethylaminopyridine catalyst in 50 ml methylene chloride, cooling as necessary to keep the reaction temperature <25 °C. When the addition was complete the mixture was stirred 1 h at room temperature, producing a pale brown, slightly cloudy solution. The solution was diluted with 100 ml hexane, and filtered through a pad of silica gel, rinsing the filter pad with 2:1 hexane in methylene chloride. The resulting clear solution was concentrated to a white solid which was taken up in ether and used immediately.
Butyllithium (2.24 M in hexanes) was added to an ice-bath cooled solution of 1-undecyne (2.28 g, 15 mmol) and ~50 mg triphenylmethane indicator in 50 ml dry THF under Ar until a pink color persisted (~7 ml, 15.7 mmol). The solution was stirred for 1 h, then the ether solution of the triflate was added dropwise at 0 °C, and the mixture was warmed to room temperature and stirred overnight. The reaction was then quenched with saturated aqueous NH4Cl, and extracted with hexane. The hexane layer was washed with brine, dried, and concentrated. The residue was flushed through a pad of silica gel with hexane, then Kugelrohr distilled (oven temp ~50 °C, 0.2 mm Hg) to remove excess 1-undecyne. The solid residue was then recrystallized from 50 ml acetone, warming to solubilize the product, then cooling to room temperature, yielding the alkyne product as a single peak (1.76 g), with additional impure alkyne in the filtrate.
The alkyne (1.7 g) was taken up in 30 ml hexane, and quinoline (0.75 ml) and Lindlar catalyst (75 mg) were added. The reaction flask was sealed and flushed sequentially with nitrogen, then hydrogen, then connected to a gas burette filled with hydrogen. The mixture was stirred until hydrogen uptake ceased. After flushing with nitrogen, the mixture was filtered through celite, most of the quinoline was removed under high vacuum, and the residue was recrystallized from 30 ml of hot acetone, after cooling to 4 °C. The resulting white solid was still contaminated with quinoline, and so a hexane solution was flushed through a plug of silica gel with hexane. After concentration, the residue was recrystallized again from acetone, yielding the alkene as a white solid (1.64 g, 98.7% pure by GC). EI Mass spectrum (m/z, abundance): 462 (12), 434 (1), 390 (1), 376 (1), 362 (1), 348 (1), 334 (1), 320 (1), 306 (1), 292 (1), 278 (1), 264 (2), 250 (2), 236 (1), 222 (2), 208 (3), 195 (3), 181 (4), 167 (6), 153 (9), 139 (14), 125 (30), 111 (54), 97 (97), 85 (37), 83 (84), 71 (54), 69 (69), 57 (100), 55 (74), 43 (90), 41 (43).
Hygienic response to treatment of pupae with Z10-C33
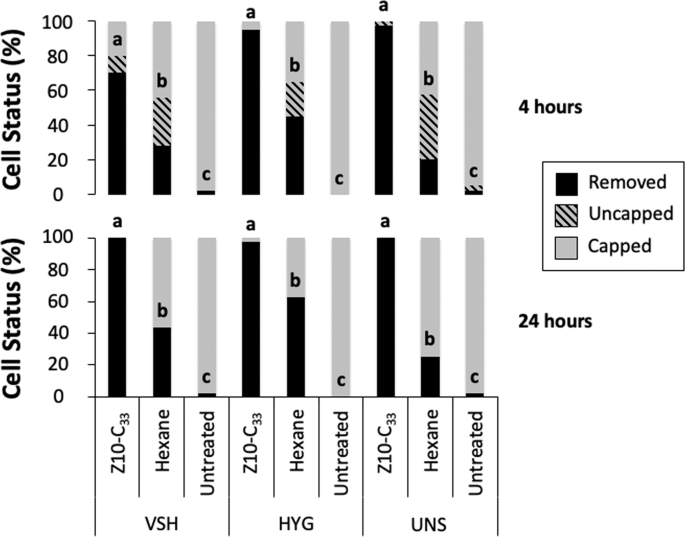
This experiment was conducted using one VSH, one HYG, and one UNS colony. To obtain a same-age cohort of honey bee brood, the locations of uncapped brood cells containing 5th instar larvae were marked using a permanent marker on transparent plastic sheets secured above experimental cells with thumbtacks. Combs containing experimental cells were placed back into the colony and recollected within 8 h. Cells capped within that time were marked for experimental use, and frames were returned to their respective colonies. On day 6 post-capping, experimental cells were opened by cutting and lifting one side of the cell cap with a razor blade. The pupa underneath received either no treatment, or treatment with either 1 μL of hexane or with 1 μL of 1.0% Z10-C33 in hexane. Cells were then resealed by gently pressing the cap against the cell wall with the side of a razor blade, and frames were returned to their respective colonies. Uncapping and removal of brood in experimental cells were recorded at 4 and 24 h after the frame was reintroduced. Sample sizes were 40 cells per treatment for UNS and HYG colonies, and 50 cells per treatment for the VSH colony.
Effects of treatment of pupae with Z10-C33on development
This experiment was conducted using the same three VSH, HYG, and UNS colonies. As described above, capped cells containing brood 6-d post-capping were carefully opened, and the brood inside received either no treatment, or treatment with either 1 μL of hexane, or 1 μL of 1.0% Z10-C33 in hexane. Pupae were then removed from the brood comb using flexible-tipped forceps, and gently placed onto fan-folded filter paper in Petri dishes. Petri dishes were placed in an incubator maintained at 34 °C and 50% RH. Brood was examined for injury (dark pigmentation) after 48 h in the incubator, and any injured brood were discarded. On the day after expected emergence, brood were examined for normal development, defined by typical adult pigmentation and shape with proper wing development. Any deviation from this was considered “deformed” and used to calculate developmental success. Brood sample sizes were 35, 29, and 27 individuals per treatment for VSH, HYG, and UNS colonies, respectively.
Hygienic response to wax cap treatment
We tested effects on hygienic behavior of application of test compounds to wax caps of brood cells. Hygienic assays were conducted by applying hexane, 0.1%, 0.3%, or 1.0% dilutions of Z10-C33, Z6-C15, or appropriate controls (Z16-C32 and Z7-C15, respectively) in hexane to capped brood cells in a VSH colony. For each assay, 2 mL of solution were applied to a circular area of capped honey bee brood. Similar to the established freeze-killed brood assay68, the treated area was isolated using a piece of PVC pipe (7.5 cm inner diameter, approximately 8 cm long). Chemicals were applied using an H-100D Single Action airbrush and compressor (Paasche, Kenosha, WI), modified with glass bottles fitted with glass tubing for this application. For each assay, 2 mL of the solution were added to the bottle immediately before application to wax caps. Thus, 0.1%, 0.3%, and 1.0% solutions deposited approximately 45, 136, and 453 µg hydrocarbon/cm2 of capped honey bee brood, respectively. Capped cells were counted directly after treatment, and frames were returned to the colony. After 24 h, frames were recollected, and capped cells in the treated region were recounted. For comparisons of Z10-C33, Z16-C32, and hexane, sample sizes were 8, 6, and 3 replicates, respectively. For comparisons of Z6-C15, Z7-C15, and hexane, sample sizes were 12, 6, and 5 replicates, respectively. Because brood availability was limited, priority was given to replication of Z10-C33 and Z6-C15 assays, followed by assays of the structural controls Z16-C32 and Z7-C15. Assay scores were determined by dividing the total number of uncapped and removed cells after 24 h by the total number of capped cells in the circular assay area at the beginning of the assay.
Effects of wax cap treatment on development
Capped VSH brood cells in a 7.5 cm diameter circular area were left untreated (control) or treated with 2 mL of hexane, 0.1%, 0.3%, or 1.0% Z10-C33 or Z6-C15 solutions in hexane with the airbrush, as described above. After 30 min, white-eye pupae (aged 5-6 days post-capping) were removed from the brood comb using flexible-tipped forceps, and gently placed onto filter paper in Petri dishes held in an incubator (34 °C, 50% RH). Brood was examined for injury (dark pigmentation) after 48 h in the incubator, and any injured brood were discarded. On the day after expected emergence, brood were examined for normal development, as described above. Any deviation from this was considered “deformed” and the proportion of successful development calculated. Brood sample size was 40 pupae per treatment.
Comparison of freeze-killed brood (FKB) and Z10-C33-treatment assays
Hygienic responses to Z10-C33 wax cap treatment and FKB assays were compared in ten colonies, including 2 HYG, 2 VSH, and 6 UNS. For the chemical assay, 1.0% Z10-C33 was applied to wax caps as described above, and any uncapping or removal of a cell after 24 h was counted as a cell targeted by hygienic behavior. The percentage of such cells among all initially capped cells was calculated for the assay score, as above. For FKB assays, a 7.5 cm diameter PVC tube was placed on a section of capped pupae aged 3-10 d post-capping, and brood in the assay area were frozen using liquid nitrogen. Frames were returned to their colony of origin, and after 24 h, all cells within the test area that still contained any pupae were counted and recorded, according to standard practice68. Assay scores were determined by dividing the total number of cells containing any pupae after 24 h by the total number of capped cells in the circular assay area at the beginning of the assay and subtracting this number from 1.
Statistical analyses
Pearson’s Chi-square analysis with Bonferroni correction was used to test effects of pupal treatment on hygienic responses in VSH, HYG, and UNS colonies. All analyses were comparisons of manipulated (uncapped or removed) versus non-manipulated (capped) cells. Pearson’s Chi-square analysis with Bonferroni correction was also used to test effects of pupal treatment on the numbers of successfully and unsuccessfully developing VSH, HYG, and UNS brood. Two-way ANOVAs with Bonferroni-corrected post-hoc comparisons were used to test effects of chemical type and chemical concentration on hygienic response to wax cap treatments. Pearson’s Chi-square analysis with Bonferroni correction was used to test effects of wax cap treatment on the development of VSH, HYG, and UNS brood. A Pearson’s correlation coefficient was calculated to test for a positive correlation between hygienic responses to Z10-C33 and FKB assays across colonies, and a one-tailed p-value is reported. Chi-square analyses were calculated based on raw data, while assay scores and related analyses were based on percentages. All statistics were performed using IBM SPSS Statistics, Version 25.
Source: Ecology - nature.com


