Material
The ice samples used for this study come from bag samples allocated to gas consortium (A cut) of the EPICA Dome C 2 ice core. They were stored after drilling (2002-2003) in France (Le Fontanil) at −20 °C before being transported to LSCE, Gif sur Yvette in December 2017 for analysis in first semester of 2018. In total, 53 ice samples were analysed but 3 samples were lost due to an excess of pressure in the line.
Extraction of air trapped in bubble from ice
Extraction of air trapped in bubbles of ice was performed using a semi-automatic extraction and separation line. A 5-mm-thick section of ice was removed from each sample prior to measurement to prevent any contamination from ambient air or drilling fluid. The sample is then introduced in a frozen flask (−20 °C) attached to the extraction line together with 5cc flasks of outside air (standard for isotopic composition of oxygen). For each daily sequence, two flasks of exterior air and three duplicated ice samples (i.e. 6 ice samples) were analysed. After pumping the line 40 min with samples kept at −20 °C, the ice samples are melted to extract the air trapped in the bubble from the melted ice. The air then goes through H2O and CO2 traps immersed in ethanol cooled at −90 °C and in liquid nitrogen (−196 °C) respectively. The gas sample is transferred in a molecular sieve at −196 °C, then in a chromatographic column (1 m*2 mm) to separate O2 and Ar from N2 and finally goes through another molecular sieve to purify O2 and Ar from remaining He. The extracted air is then trapped in stainless-steel tubes in an 8 port collection manifold immerged in liquid helium (4.13 K). The method follow description from Barkan and Luz57 except that the chromatographic column is longer (1 m instead of 20 cm) and kept at higher temperature (0 °C instead of −90 °C).
Mass spectrometer measurements
Measurements of 18O/16O, 17O/16O and 32O2/40Ar (δO2/Ar) were performed using a multi-collector mass spectrometer Thermo Scientific MAT253 run in dual inlet mode. For each extracted air sample, 3 runs of 24 dual inlet measurements were performed against a laboratory standard gas obtained by mixing commercial O2 and commercial Ar in atmospheric concentration. The mean of the three runs were calculated for each sample and the standard mean deviation is calculated as the pooled standard deviation over all duplicate samples hence integrating the variability associated with extraction, separation and mass spectrometry analysis.
The resulting pooled standard deviations before the corrections are 0.05‰, 0.02‰, 6.4‰ and 6 per meg for δ18Oatm, δ17Oatm, δO2/Ar measurements and Δ17O of O2 calculation, respectively.
Atmospheric air calibration
Every day, δ18O, δ17O and δO2/Ar of atmospheric air are measured and are then used to calibrate our measurements following:
$$delta ^{18}{mathrm{O}}_{{mathrm{ext}},{mathrm{air}},{mathrm{corr}}} = left[ {frac{{left( {delta ^{18}{mathrm{O}}_{{mathrm{sample}}}/1000} right) + 1}}{{left( {delta ^{18}{mathrm{O}}_{{mathrm{ext}},{mathrm{air}}}/1000} right) + 1}} – 1} right]ast , 1000,$$
(1)
$$delta ^{17}{mathrm{O}}_{{mathrm{ext}},{mathrm{air}},{mathrm{corr}}} = left[ {frac{{left( {delta ^{17}{mathrm{O}}_{{mathrm{sample}}}/1000} right) + 1}}{{left( {delta ^{17}{mathrm{O}}_{{mathrm{ext}},{mathrm{air}}}/1000} right) + 1}} – 1} right]ast , 1000.$$
(2)
The δ18Oext air and δ17Oext air were constant during the two measurement periods so that we used the average values over the two corresponding periods to correct the raw data. The daily correction was the same every day during each period.
Correction due to fractionation in the firn column
Several corrections were applied on the measured data due to fractionation in the firn. We describe here corrections due to gravitational and thermal fractionation as well as pore close-off effect.
Gravitational fractionation operates in firn due to Earth gravity field. δ18O and δ17O were obtained by corrections for this diffusive process using δ15N in neighbouring samples58. The correction applied depends on the difference of mass between the two isotopes considered so that:
$$delta ^{18}{mathrm{O}}_{{mathrm{gravitational}},{mathrm{corr}}} = delta ^{18}{mathrm{O}}_{{mathrm{measured}}} – 2, ast , delta ^{15}{mathrm{N}},$$
(3)
$$delta ^{17}{mathrm{O}}_{{mathrm{gravitational}},{mathrm{corr}}} = delta ^{17}{mathrm{O}}_{{mathrm{measured}}} – 1, ast , delta ^{15}{mathrm{N}}.$$
(4)
Diffusive processes operating in the firn column due to changes in temperature or because of the Earth gravity must also be taken into account. These processes lead to isotopic fractionation of O2 that was taken into account for δ18Oatm reconstruction in the NGRIP ice core over abrupt temperature changes of the last glacial period59. To check the effect of thermal fractionation on Δ17O of O2, we performed measurements of Δ17O of O2 over the top 17 m of the EastGRIP firn were a strong seasonal gradient is present. However, the resulting Δ17O of O2 was not showing any significant deviation from the atmospheric value hence suggesting that thermal fractionation does not modify the Δ17O of O2 of the atmosphere in the firn column (Supplementary Table 3). Moreover, in Antarctica, surface temperature variations are much lower than in Greenland during deglaciations or climatic variability of the last glacial period so that thermal fractionation is not expected to have a significant effect on the isotopic composition of trapped oxygen.
Pore close-off at the bottom of the firn has been shown to affect δO2/N2, δAr/N2 with potential effects on δ15N and δ40Ar in certain cases60. We checked this possible effect on Δ17O of O2 by comparing Δ17O of O2 in bubbly ice at the top of the NEEM ice core. After correction of gravitational effect, we found a systematic enrichment of 13 per meg which could potentially bias the reconstruction of atmospheric Δ17O of O2 from Δ17O of O2 in trapped air.
Gas loss correction
During storage, O2 in ice samples is subject to gas loss fractionation due to diffusion processes61 and O2/N2 ratio is always lower by several % in ice samples stored several years at −20 °C than ice samples stored at −50 °C62. Such gas loss effect is also associated with isotopic fractionation of oxygen, δ18Oatm trapped in the ice being higher when δO2/N2 decreases with a slope for the relationship of −0.01 (δ18Oatm vs δO2/N2)17,18,63,64. We thus expect that δ17O can also be affected by this gas loss process and that it may create an anomaly of Δ17O of O2. To check this effect, measurements of δ17O, δ18Oatm, Δ17O of O2 and δO2/Ar have been performed on three samples of GRIP ice core (clathrate ice stored during more than 20 years at −20 °C). Each of the three ice samples have been cut in order to analyse the interior and the exterior of the sample separately. δO2/N2 measurements could not be performed on exactly the same samples so that we used the δO2/Ar measurements to estimate the amount of oxygen loss. Argon is also known to be affected by gas loss17,61 but in a smaller extend than oxygen65 so that the decrease of δO2/Ar is still a good indication of larger gas loss.
Data show a systematic lower Δ17O of O2 value in the exterior sample compared to the interior sample, paralleling the lower δO2/Ar value in the exterior sample compared to the interior sample as expected by gas loss (Supplementary Table 1). This systematic relationship and the Δ17O of O2 and δO2/Ar values can be used to propose a correction for the Δ17O of O2 that takes into account the gas loss effect:
$$Delta ^{17}{mathrm{O}}_{{mathrm{gas}},{mathrm{loss}},{mathrm{corr}}} = Delta ^{17}{mathrm{O}}_{{mathrm{sample}}} – 0.3945 , ast , left[ {left( {delta {mathrm{O}}_2/{mathrm{Ar}}} right)_{{mathrm{sample}}} – left( {delta {mathrm{O}}_2/{mathrm{Ar}}} right)_{{mathrm{std}}}} right].$$
(5)
Comparison of EDC Δ17O of O2 with previous Δ17O of O2 record
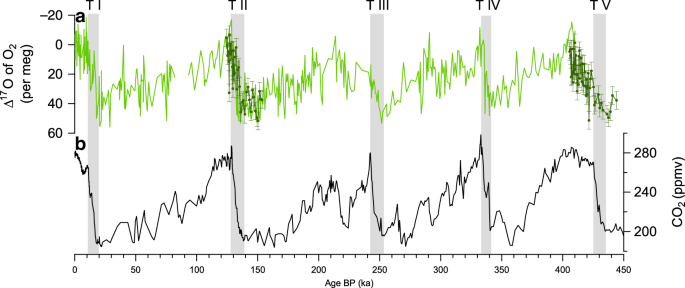
Previous Δ17O of O2 measurements only took into account correction linked with gravitational fractionation. As a consequence, we checked the consistency of previous records performed on the GISP2 and Vostok ice core with our new EDC data by measuring again Δ17O of O2 over Termination II using EDC samples and integrating the aforementioned correction (Fig. 1).
Flux of oxygen associated with biosphere productivity
We follow here the calculation of the flux of oxygen associated with biosphere productivity described in Landais et al.33.
Since the atmospheric Δ17O of O2 (Δ17Oatm) is influenced by the exchanges with biosphere and stratosphere, it is possible to write the following equation in a 3-box system at equilibrium (biosphere—bio-, atmosphere—atm-, stratosphere—strat-):
$${{F}}_{{mathrm{bio}}} , ast , left( {{Delta}^{17}{mathrm{O}}_{{mathrm{bio}}} – {Delta} ^{17}{mathrm{O}}_{{mathrm{atm}}}} right) = {{F}}_{{mathrm{start}}} , ast , left( {{Delta} ^{17}{mathrm{O}}_{{mathrm{start}}} – {Delta} ^{17}{mathrm{O}}_{{mathrm{atm}}}} right),$$
(6)
where Fbio is the flux of oxygen exchanged by photosynthesis and respiration (both fluxes being considered at equilibrium) and Fstrat is the flux of oxygen exchanged between the lower atmosphere (or troposphere) and the stratosphere (fluxes in and out of the stratosphere are assumed equal).
To reconstruct past biospheric fluxes between terrestrial/oceanic biosphere and atmosphere based on Δ17O of O2, it is necessary to know the evolution of the stratospheric flux as well as of the Δ17Ostrat. Luz et al.20 and Blunier et al.22 showed that a good assumption is to consider that the production rate of depleted O2 in the stratosphere can be related to the atmospheric CO2 concentration. It is then possible to express the evolution of biosphere oxygen flux in the past compared to pre-industrial from the following equation:
$$frac{{{{F}}_{{mathrm{bio}},{mathrm{t}}}}}{{{{F}}_{{mathrm{bio}},{mathrm{pre}}{mbox{-}}{mathrm{industrial}}}}} = frac{{({mathrm{CO}}_2)_{mathrm{t}}}}{{({mathrm{CO}}_2)_{{mathrm{pre}}{mbox{-}}{mathrm{industrial}}}}}, ast , frac{{{Delta}^{17}{mathrm{O}}_{{mathrm{bio}},{mathrm{pre}}{mbox{-}} {mathrm{industrial}}}}}{{{Delta}^{17}{mathrm{O}}_{{mathrm{bio}},{mathrm{t}}} – Delta ^{17}{mathrm{O}}_{{mathrm{atm}},{mathrm{t}}}}},$$
(7)
where Fbio,t is the biosphere oxygen flux at a given time, Fbio,pre-industrial is the present time biosphere oxygen flux, (CO2)t and (CO2)pre-industrial correspond to the atmospheric CO2 concentration at a given time and at the pre-industrial period respectively, Δ17Obio,pre-industrial and Δ17Obio,t are the values of Δ17O of O2 in an atmosphere that would only be influenced by exchanges with the biosphere at the present time and at a given time respectively. Δ17Oatm, t is the Δ17O of O2 of the atmosphere at a given time measured in the air trapped in ice core. We refer to pre-industrial period because of the long residence time of oxygen in the atmosphere (>1000 years), Δ17O of O2 is not exhibiting any significant variation over the last centuries.
Δ17Obio can be calculated from the fractionation coefficients associated with the different processes leading to oxygen fluxes in the biosphere (mainly photosynthesis, dark respiration and photorespiration). Detailed calculations of Δ17Obio at the LGM and pre-industrial periods were obtained in Landais et al.33 taking into account uncertainties in the determination of the fractionation coefficients34 as well as on the relative fluxes of oxygen (Supplementary Table 2).
From the calculation of Δ17Obio, pre-industrial and Δ17Obio,LGM (Supplementary Table 2), Δ17Obio,t is calculated through a scaling on the variations of CO2 concentration between pre-industrial period (280 ppmv) and the LGM (190 ppmv)66 such as
$$Delta ^{17}{mathrm{O}}_{{mathrm{bio}},{mathrm{t}}} = Delta ^{17}{mathrm{O}}_{{mathrm{bio}},{mathrm{pre}}{mbox{-}}{mathrm{industrial}}} + left( {{Delta}^{17}{mathrm{O}}_{{mathrm{bio}},{mathrm{LGM}}} – Delta ^{17}{mathrm{O}}_{{mathrm{bio}},{mathrm{pre}}{mbox{-}}{mathrm{industrial}}}} right), ast , left( {frac{{280 – left( {{mathrm{CO}}_2} right)_{mathrm{t}}}}{{90}}} right).$$
(8)
Details on uncertainty in photosynthesis fractionation
We performed sensitivity tests to compare biosphere productivity reconstructions without any fractionation and reconstruction with the different fractionation effects during marine photosynthesis as observed in Eisenstadt et al.37. The largest change on the reconstructed biosphere productivity is obtained using the observed photosynthesis fractionation effect associated with Emiliania huxleyi (δ18O = 5.81‰ and slope between ln(δ17O + 1) and ln(δ18O + 1) of 0.5253). The reconstructed global biosphere productivity is lower with such fractionation effect: the MIS 11 productivity level is decreased by 3% with such fractionation effect compared to the reconstruction with no fractionation at photosynthesis (i.e. the average situation, see Supplementary Table 2).
Details on uncertainty in respiration fractionation
When taking into account the maximum effect, i.e. a decrease of 0.005 for the slope of the relationship between ln(δ17O + 1) and ln(δ18O + 1) during respiration, we end up with a decrease of Δ17Obio by 65 ppm. The resulting biosphere productivity reconstruction is about 16% higher during MIS 11 than the average situation (Supplementary Table 2) leading to an anomalously high O2 flux associated with gross primary productivity during Termination V.
Validation of the biosphere productivity reconstruction
The three sensitivity tests displayed (uncertainty on photosynthesis fractionation, on respiration fractionation and on fractionation within the water cycle) suggest that the biosphere productivity during MIS 11 is significantly higher than for our current interglacial, leading to the envelop displayed on Supplementary Fig. 2. Actually, explaining the Δ17O of O2 anomaly without invoking a significant increase of the global productivity at the beginning of MIS 11 requires huge change of the Δ17O of O2 produced by terrestrial biosphere (through changes in fractionation factor or changes in water cycle, hypothesis 1) or of the ratio of oceanic to terrestrial biosphere productivity (hypothesis 2). These two possibilities are rather unrealistic as detailed below.
In hypothesis 1, an increase of 30 ppm for Δ 17O of O2 produced by terrestrial biosphere is needed. This can be obtained either by a huge change in the fractionation factors between our current interglacial and MIS 11 (which is not realistic since these are based on physical processes which do not vary with time) or by a change of water cycle during MIS 11 with respect to present-day value. To reach this 30 ppm increase through modification of the water cycle, one option is to increase the 17O-excess of meteoric water by 30 ppm during MIS 11 compared to our current interglacial. We do not have 17O-excess values for MIS 11 yet, but ice core 17O-excess values obtained for the last interglacial are very similar to values obtained for the present interglacial39. Moreover, increasing 17O-excess by 30 ppm would require unrealistic decrease of relative humidity at evaporation (by 30%, Barkan and Luz67) during MIS 11 compared to our present interglacial. Finally, an increase of the mean δ18O and δ17O of meteoric water used by the plant along the meteoric water line (i.e. without global change in the global 17O-excess) would as well be a solution (see Fig. 4 of Landais et al.33) but to reach the expected increase of 30 ppm in Δ 17O of O2 produced by the terrestrial biosphere, it would require an increase of global mean δ18O of meteoric water by ~3 ‰ with respect to present-day that will be transmitted to the global δ18Oatm. The δ18Oatm during MIS 11 is between 0 and 0.5 ‰ higher than during MIS 1 so that it does not support this hypothesis.
Hypothesis 2 requires an increase of the oceanic vs terrestrial productivity by a factor of 2 during MIS 11 compared to present-day value. This does not go along available observations suggesting an increase in the ratio of terrestrial to oceanic productivity during MIS 11 (see main text) and is still well above the uncertainty for the variation of the ratio of oceanic to terrestrial productivity during our interglacial period (14%, Supplementary Table 2).
An additional validation of our reconstruction of exceptional productivity during the beginning of MIS 11 comes from the comparison of our reconstruction with the oxygen biosphere productivity variations obtained by Blunier et al.22 over the last four terminations using a different vegetation model and different formulation of the link between atmospheric CO2 and flux of Δ17O of O2 anomaly from the stratosphere. Indeed, in our approach, we directly dealt with Δ17O of O2 anomaly in the box model while it may be more appropriate to deal with the δ17O and δ18O of O2 values as done in Blunier et al.22 and suggested by Prokopenko et al.68. We thus compared our reconstruction with the one performed with the model of Blunier et al.22 (Supplementary Fig. 2). The smaller difference in the oxygen flux associated with oxygen productivity between glacial and interglacial periods in Blunier et al.22 mainly comes from the different formulations of the dependency of the stratospheric anomaly Δ17O of O2 to CO2 atmospheric concentration. Another difference comes from the variations in the triple isotopic composition of oxygen in water between glacial and interglacial periods not taken into account in Landais et al.33. We took this possible variation into account in our approach through the third sensitivity test explained above. We ran here the model of Blunier et al.22 over the new Termination V data. The comparison between the two methods of reconstruction (Landais et al.33 vs Blunier et al.22) is significant. The biosphere productivity increase at the end of Termination V is less important using the Blunier et al.22 approach than using the Landais et al.33: 10% larger than for other interglacial periods following Blunier et al.22 instead of 20% on average following Landais et al.33. Still, both approaches show that the biosphere productivity increase over Termination V is exceptional compared to the younger terminations.
Source: Ecology - nature.com


