We used biologging data from sound and movement recording tags40 on beaked whales, together with data reported in the literature, to quantify the predator abatement benefit of two aspects of their behaviour: (i) diving and vocal coordination, and (ii) ascent swimming.
Coordination
Killer whales are large brained and muscular predators with a limited diving capacity25,41. Although they can take fish from fishing lines up to 1000 m depth42, biologging data suggest that they spend most of their time at <20 m depth41. Further, the protracted and intense pack hunting effort required for killer whales to subdue cetaceans21, and their restricted ~10 min dive duration41 strongly suggest that they can only hunt mammals at or near the sea-surface. We therefore propose that deep waters are a refuge where beaked whales are safe from killer whale attacks, and we predict that groups of beaked whales will coordinate their sound production and movements so as to minimise acoustic and visual detection when abandoning the deep refuge to return to the surface. We used two sources of data to test this notion: pairs of whales in the same social group were tagged simultaneously in three instances (two Cuvier’s pairs and one pair of Blainville’s) giving a complete quantification of their spatial relationship and coordination. Tagged whales were adults or subadults of both sexes swimming in larger social groups and we assume that their behaviour is a random sample of the behaviour of other group members. These data were supplemented with an analysis of movement patterns and inferred group vocal behaviour for a larger set of whales tagged individually, as well as an extensive dataset of sightings of both species.
Using the data from paired tags, we analysed coordination in deep dives while the whale pairs remained in the same group. For each whale pair we identified the deep dives (i.e., >500 m) performed by the first tagged whale. For each such dive we then found the deep dive with closest start time performed by the second whale. This resulted in 10 deep-dive pairs for analysis (Fig. 1) with each deep dive of both whales associated with just one dive-pair. For these pairs of dives, the dive overlap, i.e., the proportion of the longest dive in each pair during which the other whale is also diving, averages 99% (SD 0.3%), while the overlap in the vocal phase, i.e., the part of the dive in which regular echolocation sounds are made, is 98% (SD 4%) (Table 1). One of the tagged pairs was in a group of Cuvier’s in the Azores which was observed to split after 9 hours, separating the tagged whales. The dive cycle recorded after this whale pair split had a dive overlap of only 29% while vocal overlap disappeared completely (Fig. 1). Table 1 reports the results of dive coordination for all whale pairs. For the Azores whale pair, the results are reported separately for the time before and after the whale pair split because from this point on the two whales were not in the same social group.
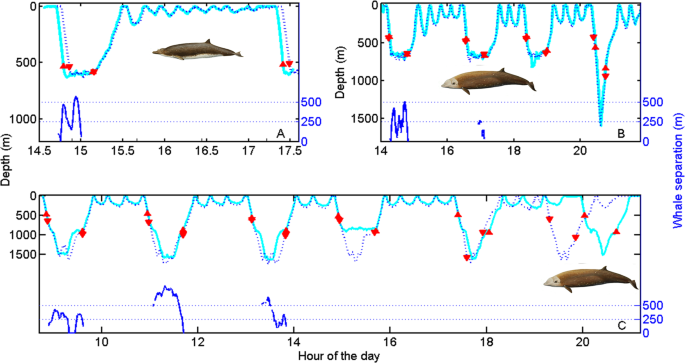
Dive profiles of three pairs of beaked whales tagged simultaneously in the same social group: (A) Blainville’s, Canary Islands; (B) Cuvier’s, Ligurian Sea and (C) Cuvier’s, Azores. For each pair, dive profiles are represented by cyan solid and blue dashed lines. Up and down red triangles mark when the first and second whale of the pair starts and finishes vocalising. The remarkable synchronization of dive and vocal activity of the whales while in a group (the Azorean group were observed to split at about 19:00), results in the whales being silent, and therefore largely undetectable by predators that rely on passive acoustics, some 80% of the time. Blue lines at the base of some dives indicate whale separation where this could be calculated from the travel time of the clicks emitted by one whale to the tag carried by its companion. Whales separate horizontally, and in one case vertically, by several hundreds of metres at the base of dives indicating individual foraging despite tight alignment of dive duration, ascent rate and vocal interval. Whale drawings by Brett Jarrett.
To test if the tight synchronization observed in whales swimming in social groups could simply be a consequence of the highly stereotyped dive cycles of these species, we analysed the overlap between the real dive profile of one whale of each pair and a simulated profile obtained from the other whale by permuting its dive cycles (i.e., each deep dive plus the following series of shallow dives before the next deep dive). This analysis excluded the Blainville’s pair that only completed one dive cycle. All permutations of the two Cuvier’s whale pairs had lower dive and vocal overlap than the actual profiles, with permuted averages of 64% and 54% overlap, respectively. Close dive and vocal timing in whale pairs is therefore the result of active coordination among group members. This interpretation is supported by the immediate loss of coordination when the Azores whale pair split. It is also corroborated with a larger dataset by examining the vocal overlap of group members audible in tag sound recordings from 12 Blainville’s (46 deep dives) tagged separately in the Canary Islands (Table 2). The low ambient noise in this field-site means that presence/absence of echolocation clicks of group members can be reliably inferred. On average, group members began and ended clicking in deep dives within 1.8 (SD 1.5) and 0.9 (SD 1.0) min, respectively, of the tagged whales, giving an average vocal overlap of 99% of the mean 26 min long vocal phase of these dives. Thus, groups of beaked whales closely coordinate their deep dives resulting in almost complete overlap of the approximately five hours per day in which they produce sound to forage. This high vocal coordination means that groups of beaked whales are available for passive acoustic detection by eavesdropping killer whales less than 25% of the time, practically independent of group size.
A consequence of the close diving coordination is that beaked whales must limit their foraging durations to match those of other group members with potentially lower diving capacity, thereby reducing individual foraging efficiency. Beaked whales live in fission-fusion societies and form groups of individuals of different age groups and sizes which nonetheless coordinate their diving and surfacing times. Even young beaked whales are observed to dive along with adults: in 18 years of field observations in the field-site of El Hierro, Canary Islands, comprising some three thousand sightings of Blainville’s and Cuvier’s groups, young have consistently been observed to dive and re-surface in close coordination with adults. This is in contrast with other deep diving species that leave young at the surface under alloparental care of group members43,44 or whose calves perform shorter shallower dives, or both45.
The impact of coordinated diving on foraging efficiency might be less severe if beaked whales hunted as a pack, e.g., actively aggregating patches of deep prey. To test this possibility, we examined the separation distance during foraging dives between the three pairs of whales tagged in the same group, using the acoustic travel time of clicks between each pair to precisely track the animals. Whales were as close as 11 m (mean 154 m, SD 15 m, range 11–305) when they began echolocating at a mean depth of 450 m. They then separated by a mean of 287 m (SD 57 m, range 11–468 m) while hunting but closed in at the end of the vocal phase to a mean distance of 127 m (SD 15 m, range 28–297) (Fig. 1). These results are consistent with the diving behaviour of beaked whale groups inferred from acoustic tracking with hydrophone arrays46. Beaked whales therefore appear to separate to forage individually within dives but are constrained by the need to approach group members before they ascend to the surface in silence. Thus, beaked whale groups are in effect joined by an acoustic leash during deep dives limiting the total foraging footprint of the group to the distance over which group members can maintain acoustic contact and reunite during a carefully timed ascent. This coordination may benefit beaked whales if they monitor acoustically successful group members to guide prey search, but coordinating may also have foraging disadvantages. Beaked whales attempt to hunt some 20–30 prey per dive2,3,4,5,23. This means that a group of e.g. five whales diving in synchronicity need to find some 100-150 prey in 20-30 min of echolocation within an area defined by the detection distance of their clicks.
The consistently high diving and vocal coordination demonstrated by both tagged whale pairs and individually tagged whales within groups, covering two species and different geographical areas, strongly suggest that collective behaviour is critical for social beaked whale groups: although the obligate deep vocal foraging intervals put beaked whales at risk of detection and stalking by killer whales performing passive acoustic tracking, beaked whales are safe to vocalize while in their deep refuge and their collective diving behaviour frees them from the need to vocalize during ascents to re-join non-diving members at the surface. This is in contrast to pilot whales or sperm whales that often vocalize during ascents to mediate group reunion acoustically10,11,12,13. That beaked whales of different genera (Mesoplodon, Ziphius) show the same coordinated behaviour suggests that the coordination of diving and vocal activity in social groups may have evolved millions of years ago or has had sufficient evolutionary value as to drive convergence towards a strikingly similar strategy.
Silent ascent swimming
Although tight vocal overlap reduces the acoustic detectability of beaked whales, they nonetheless face the risk that eavesdropping predators are waiting for them when they return to the surface. Compared to terrestrial mammals that must choose between refuges and foraging, beaked whales live in a through-the-looking glass world: they are safe while making sound to forage at depths beyond the reach of killer whales but are at maximum risk when they come to the surface to breathe. Unlike most prey species47, for beaked whales foraging is not risky but breathing is.
The low-angled powered ascents that are a distinctive feature of the foraging dives of Cuvier’s and Blainville’s2 have been proposed to serve in managing decompression sickness6 but the wide variability in overall ascent vertical speed from dive-to-dive (0.3-1.1 m/s Md, 0.4-0.9 m/s Zc48) is difficult to reconcile with a physiological need for a particular decompression rate. However, low pitch angle ascent swimming confers the direct advantage that beaked whales can cover a substantial horizontal distance during their silent ascents, potentially moving them away from waiting predators. We therefore hypothesise that beaked whales will move horizontally during ascents in such a way as to make it difficult for shallow diving killer whales tracking echolocation clicks produced at depth by beaked whales to predict where will they surface. Such a strategy is only possible for beaked whales because they do not need to re-join non-diving members at the surface. For other deep diving species such as pilot whales and sperm whales, non-diving group members, including calves left alone or under alloparental care at or near the surface43,44,45, form a surface anchor to which diving animals must return. In contrast, the collective behaviour of beaked whales frees them to choose their surfacing location.
To test the predictability of beaked whale travel during ascents we estimated dead-reckoned tracks40, constructed from the pitch, roll, heading and depth data recorded by the DTAG, for 64 and 37 silent ascents of 14 Blainville’s and 10 Cuvier’s, respectively. These tracks were plotted with respect to the mean heading during the last five minutes of vocal activity before initiating the silent ascent. The resulting tracks show that beaked whales frequently adopt headings that translate them away from the surfacing position that would be predicted by an eavesdropping predator (Fig. 2C,D). On average, whales covered one kilometre horizontal distance from the point where they stopped clicking until they reached the surface (SD 430 and 710 m for Blainville’s and Cuvier’s respectively). This behaviour creates a large circular locus of potential surfacing positions that must be searched by killer whales and which they must search visually rather than using echolocation to avoid alerting their prey24.
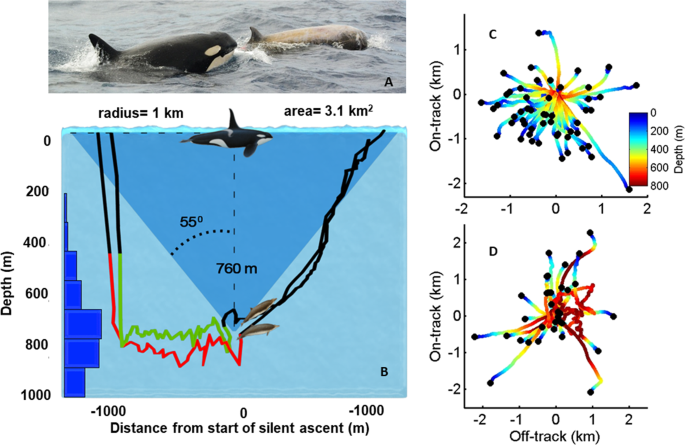
(A) Killer whales hunting a Cuvier’s beaked whale (photo by Machi Yoshida, Naturaliste Charters Australia). (B) Foraging dive tracks of two Blainville’s beaked whales tagged in the same group showing their activity synchronization. Coloured segments indicate hunting by echolocation whereas black segments indicate silent travel. Blue bars on the left show the depth distribution of all clicks from 14 tagged Blainville’s beaked whales, confirming that they are silent at depths shallower than 200 m where killer whales restrict most of their dives. Cuvier’s and Blainville’s beaked whales begin a silent ascent at a mean of 760 m depth and ascend with a shallow angle (mean 35° from the horizontal)2,48 in an unpredictable but coordinated direction. Dead-reckoned tracks show that ascending whales cover on average of 1 km horizontal distance from where they stopped clicking until they reach the surface, as represented schematically by the blue cone in panel B. (C,D) Horizontal dead-reckoned travel paths (coloured lines) of ascending Blainville’s and Cuvier’s beaked whales, respectively, with respect to their swimming direction before silencing. Travel in the same direction as the animal was moving prior to silencing is represented by the positive on-track axis in these plots while movements orthogonal to this are represented by the off-track axis. Surfacing positions (black dots) that are further from the centre of the plot are less predictable for an eavesdropping predator at the surface.
The average 1 km horizontal distance covered by beaked whales in silence during the ascent renders a surfacing uncertainty circle with an area of 3.1 km2 (Fig. 2B) which waiting killer whales must search within the 2.5 min that Cuvier’s and Blainville’s spend on average at the surface after a deep vocal dive. Assuming a swimming speed of 2 m/s for killer whales25 and a maximum visual detection range in most oceans of some 50 m underwater49, an individual killer whale can cover visually only some 1.2% of the potential surfacing area of beaked whales during a surfacing. Encounter probability increases with killer whale group size: groups of up to 12 whales have been observed attacking beaked whales21 (Fig. 2a) which could still only cover some 15% of the potential surfacing area of beaked whales with a perfectly coordinated search pattern. Thus, even if killer whales detect beaked whale echolocation clicks acoustically, the unpredictable low-angle silent ascents decrease predator encounter rate by one to two orders of magnitude compared to the vertical ascents made by other deep diving whales8,9. But for this strategy to work, and even just to maintain group cohesion, beaked whale group members must ascend with similar speed and direction without additional vocal cues. This adds to the critical importance of tight coordination at the end of the vocal foraging interval.
In the intervals between deep foraging dives, beaked whales maintain almost complete silence and so likely need occasional visual contact with other members of their social group to maintain cohesion. Between deep dives these whales typically perform a sequence of shallow non-foraging dives2,14,50, which can nonetheless reach 400 m depth and 25 min duration, and in which animals can move hundreds of metres horizontally2. We predicted that group cohesion should be evident as strong synchrony in the dive profiles of group members in the extended intervals between deep dives. To test this, we used again a permutation method on paired dive profiles. Shallow silent dives performed by the three whale pairs overlapped in duration by an average of 97% (SD 2.4, n = 29 paired dives). In contrast, the overlap of 3000 simulated shallow dive profiles (1000 per whale pair) constructed using dives randomly selected from the same whale pair, averaged just 30% (SD 4). This is consistent with field observations of Blainville’s and Cuvier’s (El Hierro), and from the tagged Cuvier pair in the Azores (Fig. 1), where beaked whale groups tend to maintain close temporal and spatial cohesion in surfacing and diving, while coordination is lost when groups split.
The costs of hiding
The collective vocal and diving behaviour of beaked whales greatly reduces both the time intervals over which groups can be detected by acoustic predators, and the post-detection interception risk, in effect enabling beaked whales to hide from eavesdropping predators. Although there may be additional benefits of close vocal and movement coordination, e.g., in sharing foraging information via mutual acoustic monitoring, as has been observed in echolocating bats51, this synchronization comes at a significant cost. The long silent ascents reduce the time available for foraging by some 35% as compared to vertical ascents, the common strategy of other deep-diving cetaceans8,9, for the same dive duration. Moreover, the closely synchronized diving behaviour must accommodate group members across a range of diving capacities, further constraining the foraging time of larger individuals. This perhaps explains the unusually large size of newborn beaked whales (approx. 50% length of the mother)52 in comparison to other toothed whales which are born at about one third of the adult size. A large birth length, and therefore weight, likely confers an advanced start for the ontogenetic development of diving capabilities and favours juveniles rapidly attaining the diving performance needed to dive with adults. Such large birth size may also explain why female beaked whales are similar in size or larger than males52 despite inter-male fights that would be expected to drive sexual dimorphism towards larger males. Similar body size may have the further benefit of harmonising diving capacity among group members reducing the cost of accommodating diverse diving endurance within a group.
Conclusions and conservation implications
The unique diving and vocal behaviour of beaked whales could only evolve if the severe costs it imposes are outweighed by survival benefits. While the natural social and diving behaviour of beaked whales may be influenced by a whole suite of physiological, life history and ecological factors, we show here that the features that make beaked whale diving and vocal behaviour distinctive compared to other toothed whales confer major quantifiable advantages in abating predation risk from killer whales and even from visual predators at the surface such as sharks. These results provide the first quantitative support for previous hypotheses that the behaviour of beaked whales is influenced by predation risk2,4,14. Thus, while sperm whales and pilot whales, aided by either size or numbers, can choose to stay and fight off killer whale attacks28,29,53, beaked whales with little social defence have adopted the strategy of hiding. Unlike many terrestrial prey species navigating landscapes of fear54 for which risk assessment is modulated temporally by perception of the state of predators and indirect predation cues15,16, beaked whales have little opportunity to assess risk, as mammal eating killer whales tend to hunt silently24 and can only be seen at short range underwater49. As a consequence, for beaked whales, tonal sounds above ambient noise that might signal killer whale presence or other threats could well provoke an anti-predator response32,33,34,35. The beaked whale strategy of hiding is borne out in their responses to sonar and killer whale playbacks: silencing and avoidance32,33,34,35. Evolution in a soundscape of fear therefore offers a mechanistic explanation for why beaked whales respond so strongly to playbacks of sonar and killer whale sounds at barely audible levels. Akin to ungulate escape responses from pursuing predators that can lead to death by physiological stress55, naval sonar that inadvertently signals a strong risk-factor, such as the sounds of apex predators, may push beaked whales beyond their physiological limits and in some cases lead to sonar induced mortalities. As such, a successful predator abatement strategy shaped by natural selection has become maladaptive in the face of novel human activities. Given the vast zones over which mid-frequency navy sonars are audible and so may impact the behaviour of beaked whales32,33,34,35, large-scale spatial avoidance of beaked whale habitats when mid-frequency sonar is used should provide the most effective mitigation measure for these cryptic species56.
Source: Ecology - nature.com



