Most studies on Müllerian mimicry have focused on behaviour, predators’ choice, and modelling, usually under controlled conditions. We suggest that the phylogeny and structure of real Müllerian communities can test experimental results and elucidate the origins of numerous patterns, their long-term coexistence, imperfect similarity, and mimetic polymorphism7,8,11,35. A similar approach has been used for studies on velvet ants36 and, here, we focus on the evolution of aposematic patterns in net-winged beetles. We base our study on the structure of Müllerian complexes and the phylogenetic analysis of the Bornean fauna, including its relationships with other Oriental Lycidae (Fig. 3A, B).
The Bornean fauna is highly endemic, and our samples contain 53 Metriorrhynchini and 5 Calochromini species, only 4 of them known from neighbouring regions. Molecular data, as well as morphological divergence, indicate that the closest relatives are present in the Malay Peninsula and Sumatra and that the divergence between species justifies species rank for most species pairs endemic to respective areas as has been shown in the earlier detailed study of the net-winged beetle faunas of the Malay Peninsula and Sumatra37,38 and similarly reported in vertebrates39. Our sampling is undoubtedly incomplete as we analysed only 53 Bornean metriorrhynchines species compared to 69 formally described. Nevertheless, our results convincingly show that high numbers of species are involved in each Müllerian complex in each locality and that they belong to various deeply rooted lineages. Due to distant relationships of Müllerian mimics in Borneo, we suppose that their similarity is a result of natural selection, not relationships (Fig. 3A, B).
We observed individuals belonging to multiple patterns in close contact in the mountain forests of the Crocker Range. Most individuals were sampled in aggregations on a limited number of shrubs and low-stratum trees in the forest. Additionally, the mountain forests in the Mt. Crocker Range represent a geographically very limited area (Fig. 1F). The area defined by 1,500 and 2,500 m contour lines covers ~ 140 km2 in Mt. Kinabalu and ~ 60 km2 in the Mt. Alab – Mt. Emas area. The mountains species are endemic to such a small range as the mountains of north-eastern Borneo are highly isolated and net-winged beetles poorly dispersing37. The occurrence in aggregations and a very small, ecologically uniform range indicate that the observed patterns coexist in contact and are not isolated in non-overlapping microhabitats as has been observed in some butterfly mimetic systems40,41.
Broadly defined, three mimetic patterns are identified in Borneo, including the mountain areas of the Crocker Range. Both tribes, Metriorrhynchini and Calochromini contained species sharing all three patterns. The brown to reddish/black pattern A (Fig. 1A, Supplementary Fig. S6) dominates in the Bornean lowlands and lower mountain elevations up to ~ 1,400 m (Fig. 1D). Although similarly coloured, as the component of the signal can be considered also the similar body size and shape which provide at least limited signalling of unprofitability when an individual is sitting on the bottom side of a leaf and is observed against clear sky21,42,43. The extent and shade of the bright part of elytra is intra- and extra-specifically variable, but most individuals have one to two-thirds of elytra brightly coloured and these individuals can be categorized as a single pattern (the colour differences are perceptible at a glance; average DeltaE 9.87). Further, we identified the presence of two unique mimetic patterns in the elevations over ~ 1,500 m (Fig. 1E, F, Supplementary Fig. S6). The mountainous yellow/black pattern B is quite close to the widespread lowland red/black pattern and some individuals have shades of red in some parts of the yellow coloured humeri (Fig. 1B and see results for La*b* CIE76 colour positions). We assume that such a pattern could evolve from the lowland forms by a simple modification of coloration. Although some intra-pattern variability is perceptible (Fig. 1), these patterns can be easily assigned to categories and serve as a warning signal for potential predators. Their signalling role is supported by the presence of co-mimics from other beetle families and insect orders43,44.
The reticulate pattern C involves, besides a colour component, also the structure of elytral costae and it is the unique, easily distinguishable distinct signal of unpalatability (Fig. 1C). All Metriorrhynchini have either four or nine longitudinal, and numerous transverse costae connecting them (Figs. 1A–C, 4A). The relationships of the species displaying the reticulate pattern show that such a pattern has to be derived from the ancestral small-cell type. Xylometanoeus and Xylobanus have four costae even if they are uniformly black or brown/black, but either the number of transverse costae is high and as a result, the cells are small and cannot be distinguished from a distance (Fig. 1A) or even when cells are slightly larger than usual, the colouration of cells does not differ from those of costae and they cannot be distinguished from species with nine costae at the first sight. The Xylometanoeus species with the reticulate aposematic pattern has a much lower number of transverse costae, the costae are covered by red pubescence, and the large cells with black bottoms are very apparent (Figs. 1C, 2). The reticulate pattern is displayed also by some Cautires species. Almost all Cautires have nine longitudinal costae, but when these beetles display the reticulate pattern, their secondary costae are reduced. Such adaptation produces relatively large cells, nevertheless, the cells are smaller than those of Xylometanoeus (Figs. 1C, 2, 4A). Our phylogenies show that the reticulate pattern is a derived trait. We may suppose a gradual evolution of increasingly conspicuous reticulate cells as transitional forms are present in the pattern A (Delta E colour distance costa/cell 23–30 in some representatives of the pattern A and 25–41 in the pattern C). The signal is primarily based on the reticulation. Even if costae and bottom differ in colouration, more apparent is their different size (compare Fig. 1A, C).
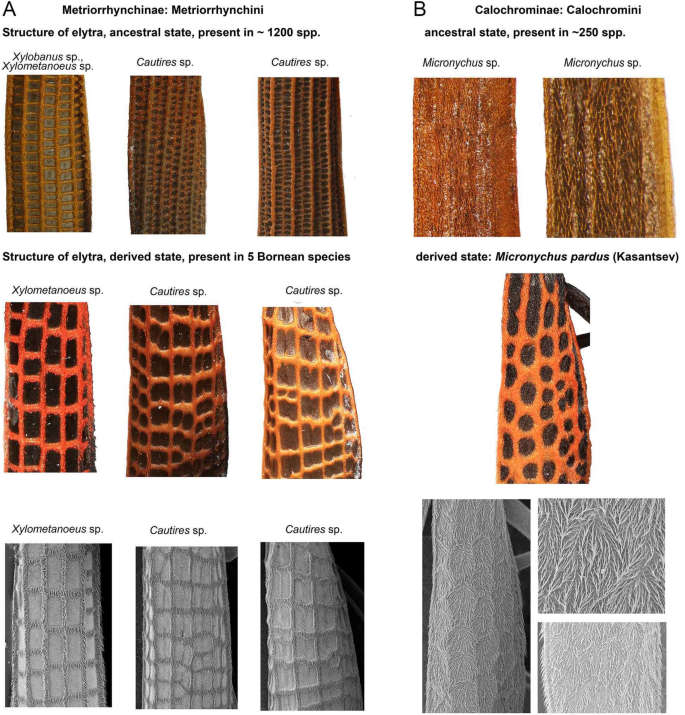
(A) The examples of the metriorrhynchine and calochromine elytral structures using light macrophotography and SEM photographs of the homological elytral parts. The genera for columns are given above, the second and third row show always the homologous parts of a single specimen. The first column shows the comparison of species with different number of transverse costae with the size of cell increased in derived forms, the second and third column show examples of the lost and/or vestigial secondary costae and increased size of cells as a result. (B) ditto of Calochromini. Above, the examples of the typical structure of costae in Calochromini. Middle and bottom, the examples of the female Micronychus pardus elytral structures using light macrophotography and SEM photographs (the ‘false’ reticulate costae resembling the pattern C).
The dated tree identified the splits between individual species displaying the focal reticulate and yellow/black patterns and their closest relatives a long time before the uplift of the Crocker Range (15–45 mya in Metriorrhynchini and ~ 15 mya in Calochromini). As each species represents a single lineage and no split between species sharing the reticulate pattern was identified, we are not able to date reliably the origin of the pattern. Better data could be potentially obtained with denser sampling, which is currently unavailable. Therefore, we must rely on indirect evidence to date the origin of patterns. The Crocker Range is a tectonically young mountain area and was uplifted in the last ~ 7.5 million. The net-winged beetles are common and diverse in high-mountain areas which obtain high rainfall, i.e., in the montane forests established in the area at the earliest 6 mya22,31,32.
The origins of high-contrast patterns in a mimetic community
The yellow/black and reticulate patterns B and C show a higher Delta E colour distance, i.e., internal contrast, than the widespread brown/black pattern A (Figs. 1B, C, 4A, B). The high-contrast patterns were observed in several species of Metriorrhynchini and a single species of Calochromini and Platerodini; other net-winged beetles do not display them. The reticulate pattern C was identified in 5 species, 4 metriorrhynchines and 1 calochromine, the yellow/black pattern B in 13 metriorrhynchine species, 1 Plateros, and 1 Micronychus (Fig. 1B, I). The phylogenetic analysis shows that all of them represent terminal lineages (Fig. 3A, B). As the reticulate and yellow/black patterns do not occur outside the north-eastern Bornean mountains, we must suppose their de novo sympatric origin within the earlier local mimetic communities which display widely distributed pattern A in Borneo as a whole and in most of south-east Asia.
The reticulate structure of elytral costae is apparently derived from patterns displayed by their closest relatives35,36. In the principle, the distinctiveness of the reticulate pattern is based on the lower number of costae (four instead of nine longitudinal costae) and a lower number of transverse costae in the length of the elytron (8–15 versus > 30 transverse costae in patterns A and C, respectively). As a result, the elytral cells are much larger, 0.29–0.48 mm2 compared to 0.10–0.26 mm2 in non-reticulate elytra (Fig. 2). Additionally, the dense pubescence of costae is bright coloured and the cell bottoms are black (Delta E 25.0–41.0 between costae and cells in the pattern C; DeltaE up to 28.0 in pattern A; Fig. 3C, J).
Similarly, the yellow and black coloured parts of elytra in the pattern B display a higher internal contrast than reddish brown/black pattern A (Delta E 52.5–69.7 in the pattern B and 15.7–22.2 in the pattern A, respectively). Therefore, as perceived by a human eye, the pattern B is more conspicuous than the dominant and widespread reddish brown/black pattern A of relative species (Figs. 1, 3, 4). Its distinctness is based on a higher internal contrast between bright and black parts due to the bright yellow pubescence and light colouration of costae and cells in the basal part of elytron (Figs. 1, 4). High contrast and structural uniqueness are principal components of the signal and increase its effectiveness13,14,15,16,35. We can expect variable perception under different levels of illumination and variable external contrast on different backgrounds (old dark coloured leaves versus young thin light green leaves). Although, the perception might vary under different conditions, the colour differences are perceptible and can be quantified.
In the contrast with expectations based on frequency dependent purifying selection6, we observe a relatively low numbers of individuals displaying the reticulate pattern C and most individuals belong to a single species of Xylometanoeus (Fig. 1C) and other four species were collected in few specimens (Table S1). Therefore, we assume that species displaying the reticulate pattern C do not have the full protection of a high number of individuals. Their relatives with whose they undoubtedly shared the widespread and very common reddish/brown pattern a few million years ago absolutely dominate in the lower elevations. The theory-based expectation is that the species displaying a less common pattern suffer a higher attack rate if they encounter uninformed predators. The disadvantage of the rareness must have been even stronger in the early phase of the evolution of these patterns when the first more conspicuous distinct forms evolved, and a low number of individuals displayed the new pattern. We have to consider some factors potentially supporting survival of such conspicuously coloured individuals and compensating the disadvantage of the low number of individuals. The only potential benefit, which the members of a new mimetic ring attained due to their new high-contrast aposematic signal, is possible predators’ rapid ability to associate unprofitability with their unique reticulate pattern and the ability to retain such association for a long time6,14,45,46,47,48. Therefore, as a working hypothesis, we assign the apparent success of such a new pattern to its high internal contrast which putatively facilitates avoidance learning and offsets the disadvantage of low numbers.
The survival of conspicuous prey unknown to local predators has been a conundrum of the mimicry theory3. The here described process suggests that it is possible to abandon an extensive mimetic ring and to sympatrically establish a new distinct pattern despite the predicted higher selection load of supposed higher attack rate per capita from local predators. Therefore, we suggest that new aposematic patterns can be potentially established in original Mullerian communities if the new aposematic signals are sufficiently strong, e.g., easily learned by local predators. As a consequence, we must additionally suppose that mimetic communities continually expand. The number of species in the Müllerian ring rises due to advergence of additional species to local patterns and by the sympatric origins of new patterns.
The intraspecific polymorphism is a possible adaptation in multi-pattern communities as has been shown recently in another metriorrhynchine genus Eniclases21, but the recovered phylogeny indicates that the acquisition of a new pattern was regularly accompanied with the speciation. In such a way, the alpha-diversity diversity was putatively generated in situ in the Crocker Range32. Probably due to a limited dispersal capacity, the conspicuous endemic patterns of net-winged beetles are restricted to very small ranges as we observe in the Crocker Range and earlier in Sumatran and Malayan mountains37. The occurrence of the new reticulate pattern is limited in the Crocker Range to an area between 1,500 m and the upper limit of the montane forest, i.e., to ~ 200 km2 (Fig. 1F). The small range and a low capacity to colonize geographically distant ecosystems are, in the long term, a disadvantage which may lead to extinction if original habitats are lost.
How to overcome constraints in multi-pattern communities
Our previous discussion focused on metriorrhynchine net-winged beetles. One of the species displaying the reticulate pattern, Micronychus pardus, belongs to the Calochromini (Calochrominae, a diverse tribe with a cosmopolitan distribution49), a distantly related subfamily whose members do not have any transverse costae (Fig. 4B). The absence of a structure that is crucial for the adoption of the local reticulate aposematic signal is undoubtedly a serious disadvantage if the adoption of such a signal would be profitable. Nevertheless, M. pardus evolved a unique reticulate structure of setae on their elytra which resembles the real elytral costae (Fig. 4B).
How did Micronychus overcome the absence of transverse costae? The ancestral morphology does not indicate the gradual accumulation of cost-free mutations over the range of imperfect signals6. As the transitional origin of small cells and their subsequent modifications would be complicated, we assume the direct evolution of a few transverse “costae” to adverge to the reticulate pattern. We intentionally designated the structure as “costae”, because, in fact, the perception of costae is merely an optic illusion caused by the different colouration and direction of dense setae on their elytra (Figs. 1J, 4B). We suppose that if a net-winged beetle which does not have any transverse costae adopts such a new reticulate aposematic pattern, the whole process must be rapid and based on the advergence to the already present reticulate signal. The transitional stage would be neither conspicuous nor known to local predators and all predictions suggest that it should be quickly wiped out. The resulting pattern is undoubtedly similar to those of the local Metriorrhynchini. Nevertheless, the similarity is limited by the ancestral morphology and at least to the learned field entomologist serving as a model predator this pattern does not seem perfect. Despite these limits, we must consider calochromine ‘false costae’ as a stunning example of the power of evolution when structure which should be modified is absent (Figs. 4B). We cannot tell anything about the mechanism leading to such a modification, but the plasticity of setal colours must be high as shown recently by Tiana et al.50.
The situation of unpalatable Micronychus pardus among other unpalatable species that use their reticulate costae as an aposematic signal seems complicated enough, but if something can become more complicated, it undoubtedly becomes. The net-winged beetles, similarly to most beetles, have quite large-bodied females and small-bodied males. We found that only females of M. pardus adopted the reticulate pattern C. The conspecific male (a single individual available) is small-bodied (male 8.1 mm versus female 9.8–12.8 mm) and surprisingly, it does not resemble its conspecific female. The male resembles yellow/black coloured metriorrhynchines34, the pattern B (Fig. 1I) and we suppose that the body size plays an important role in the signalling as shown earlier in Dilophotes net-winged beetles44.
The adaptations of sexually dimorphic M. pardus are a stunning example of a very complex evolutionary process when males and females follow different evolutionary pathways in multi-pattern mimetic communities. Both sexes adopt unique patterns within Calochromini, and the females overcome the absence of an important morphological structure. The multi-pattern communities potentially set a very selective environment that exposes its members to various challenges. Some of them, like four metriorrhynchines and one calochromine species established in north-eastern Bornean mountains a new mimetic ring of with reticulate patterns and 13 species of metriorrhynchines, one calochromine and one platerodine the yellow/black pattern. In contrast with them, many closely related species without reticulate pattern co-occur in the area and many in close contact within the same mountain ecosystems. The number of shifts to the new highly conspicuous reticulate pattern is quite low. Although not conclusively, at least fairly convincingly, we may suggest that a good part of mimicry evolution in Bornean net-winged beetles is stochastic and depends on the availability of a mutation in the right place and time. Therefore, considerable time lags can be expected if a species should adopt a considerably different aposematic signal4. The delays then contribute to the coexistence of several patterns in a single community of unpalatable beetles.
Source: Ecology - nature.com


