There could be multiple proximate causes for fluorescence in response to blue or ultra-violet wavelengths within amphibians and the exact mechanisms that produce biofluorescence in these vertebrates require further study. Biofluorescence associated with bold colors could be the result of both chemical and structural elements of the amphibian dermal chromatophore unit24. Some pigments, like pterins and carotenoids, or reflective structures containing guanine have been shown to fluoresce5,9,25. Both are present in chromatophores or elsewhere in the skin of larval and metamorphosed amphibians24,25.
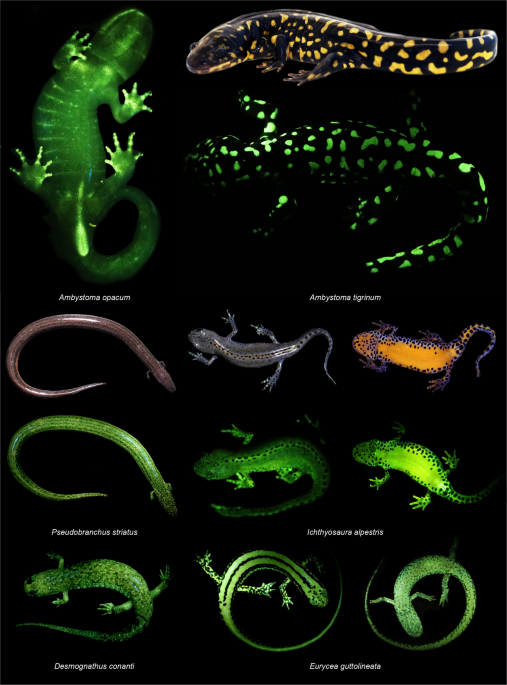
Alternatively, there could be sources of fluorescence in salamanders and other amphibians independent of their pigmentary systems. Green fluorescent proteins and their analogs are responsible for fluorescence in some invertebrates (e.g., cnidarians)1 and vertebrates (e.g., eels)2, but similar proteins have not yet been characterized from amphibians. Fluorescent compounds called hyloins have been documented from Neotropical tree frogs (Hylidae) and are associated with their lymph and glandular secretions9. Similar compounds may be responsible for the biofluorescent, mucous-like secretions which we observed in salamanders and caecilians (Supplementary Fig. 3). Recently, fluorescence in swell sharks4 in response to blue light has been attributed to a newly discovered fluorescent metabolite in their skin16. For other vertebrates, ossified elements immediately beneath the skin are responsible for biofluorescent patterns (e.g., chameleons10 and pumpkin toadlets12) under ultra-violet excitation. Here we found that the bones in the digits of the marbled salamander (A. opacum, Fig. 1) fluoresced in response to blue light.
Whether the biofluorescent light produced by an amphibian is perceived by conspecifics or heterospecifics depends in part on if individuals are active under the conditions necessary for fluorescence5. Amphibians occupy a variety of habitats, often moving between terrestrial and freshwater systems, and the ambient light environments they experience are complex17. In forests ambient light varies with the structure of the vegetative community, weather, and time of day17,18,26, but several forest types contain patches of habitat in which blue wavelengths are prevalent18. Many amphibians, including salamanders, are crepuscular or nocturnal. During twilight, the ambient spectra in terrestrial systems shifts to predominantly blue light18,26. Both light environments include wavelengths in the visible blue spectrum which we have shown result in green fluorescence (Fig. 2). Terrestrial organisms that are active on the surface during daylight and early twilight will also be exposed to ultra-violet radiation17 which can result in biofluorescence in both anurans9,12 and salamanders11. We have added three species to the list of amphibians which biofluoresce in response to ultra-violet excitation (Ceratophrys cranwelli, Ambystoma tigrinum, A. laterale) (Supplementary Fig. 2), though the intensity of fluorescence we observed was less than when exposed to blue excitation light (Figs. 1 and 2). Although our study and others have confirmed the presence of fluorescence in anurans in response to ultra-violet light, other recent work surveying for biofluorescence in Neotropical tree frogs did not identify strong emissions under ultra-violet excitation27.
Perception of biofluorescent light also depends on the sensitivity of the eye of a potential observer. Rod photoreceptors in the retina play a key role in vision during low-light conditions26 such as those during twilight. Like other vertebrates, salamanders, frogs, and caecelians have “red-rods” which are maximally sensitive to green light22,28,29,30. Biofluorescence in amphibians could potentially contribute to achromatic vision and perception of other individuals in low-light environments. Recent studies demonstrate that the unique, dual-rod system of some amphibians, which includes a “green rod” that is maximally sensitive to blue light22,31, allows for color discrimination in dim-light31 and biofluorescence may contribute to color perception in those environments for some species. “Green rods” are absent in some salamanders (e.g., some salamandrids30) but present in others (e.g., ambystomatids29).
Whether biofluorescence contributes dramatically to the overall light emitted from an organism may vary across taxa. Taboada et al.9 documented that biofluorescence in response to ultra-violet light contributes between 18 and 29% of the total light emitted from some species of Neotropical tree frogs (Family Hylidae) under natural, dimly lit conditions. In contrast, Goutte et al.12 found that biofluorescent emissions derived from bony elements in pumpkin toadlets (Family Brachycephalidae) contributed to less than 3% of the total light emitted by these anurans, and that in this case fluorescent signals were likely negated by ultra-violet light being reflected by the toadlets. In our survey, both the blue and ultra-violet light sources used were more intense than would be experienced by these organisms in situ, and the contributions from biofluorescence versus reflectance under natural conditions is in need of further study across the range of habitats amphibians occupy.
In tetrapods, as in other vertebrates, biofluorescence may function in both intra- and interspecific communication and crypsis. In flying squirrels fluorescence is hypothesized to aid in camouflage against a backdrop of lichens emitting similar fluorescent spectra, or potentially in Batesian mimicry of co-occurring predatory owls with similar biofluorescent profiles13. Biofluorescent plumage in parrots functions in sexual selection and mate choice7, whereas in anurans it may enhance the brightness of individuals making them easier to detect by conspecifics9. Biofluorescence in salamanders may serve similar functions, and salamanders with complex reproductive behaviors involving visual signals (e.g., newts [Salamandridae] and lungless salamanders [Plethodontidae]) are observed to biofluoresce in this study. Cloacal biofluorescence in some species is particularly intriguing (Fig. 1) as this region is often the target of investigative behaviors during courtship in salamanders. The peak emissions produced by amphibians observed in this study fall within the range of green light (Fig. 2), a color which would set them apart from background vegetation that fluoresces yellow or red under blue excitation light. Future ethological, anatomical, and chemical studies are needed to determine the functional roles, if any, biofluorescence serves in the biology and evolution of amphibians.
Biofluorescence is widespread and variable across Amphibia, and our findings shine a new light on how much more we have to learn about the biology of these fascinating vertebrates. Our study provides a roadmap for future efforts intent on exploring biofluorescence in amphibians, from the potential ramifications of fluorescence on their ecology, to the chemical or structural mechanisms contributing to this phenomenon, to the potential broad applications of this knowledge across scientific disciplines (e.g., developmental biology, medical fields). Biofluorescence may also prove a useful tool in documenting amphibian biodiversity in complex microenvironments. Small, cryptically colored, and/or nocturnally active species can be hard to locate among leaf litter or dense vegetation. We propose that scientists documenting the biodiversity of amphibians could use excitation light devices and filtering lenses to visualize fluorescent amphibians and improve their ability to find taxa which are otherwise difficult to detect. This could be an inexpensive and transformative approach to how we survey the biodiversity of amphibians worldwide.
Source: Ecology - nature.com



