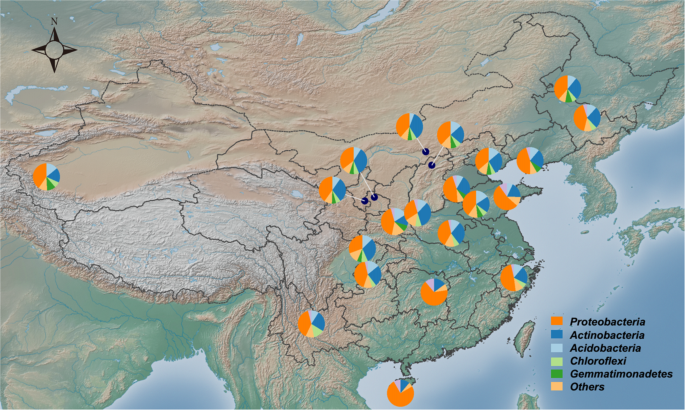
Given the global role of microbes in the environment, determining the drivers of microbial community assembly is an important issue in microbial ecology17. Microbes inhabiting agricultural soils are closely linked to crop productivity through complex biogeochemical processes18. In this study, we investigated the major environmental factors that drive bacterial community assembly in maize soils across a large spatial scale with a latitude gradient.
Maize, which possesses exceptional phenotypic and molecular diversity, can grow in diverse soils with a pH range of 5.7–8.019. In the present study, the maize soils were slightly acidic to alkaline, within the suitable pH range for growth of maize. The variation of soil pH might be related with the wide variety of soil textures. In addition, most of our soil samples (71%) were circumneutral to alkaline (pH > 7). Bacterial communities can be shaped by complex environmental factors. Significant correlations have been frequently found between the pH and bacterial alpha-diversity in soils20,21 and lake sediments22, indicating that pH is a predictor for soil microbial diversity. In the present study, OTU richness showed a marginally significant and positive correlation with soil pH in the range of 5.23–8.86, in agreement with previous observation in British soils over a broader pH range from 3 to 920. Soil pH could affect the bioavailability of carbon and nitrogen substrate as well as toxic metals, indirectly influencing soil microbes23. The richness and diversity of bacterial communities are key maintainers for the productivity and stability of agricultural soil ecosystems3.
Two climatic factors, mean annual precipitation and temperature, also predicted bacterial diversity in the maize soils. These two variables were negatively correlated with the Shannon–Wiener index of soil bacterial communities in the current study, although we also acknowledged that the significant relationship between the Shannon index and the average annual precipitation may be driven by two outliers. This result could be supported by a continental-scale study across eastern China24, which reported higher bacterial diversity at high latitudes in maize fields. However, conflicting results were reported in natural terrestrial ecosystems that soil bacterial diversity was positively or not correlated with temperature10,13. The discrepancy may be associated with the complex and heterogeneous environments across different habitats across multiple scales.
In the present study, both mean annual precipitation and soil pH were significantly linked to bacterial community structure in maize soils based on beta-diversity metrics. Similarly, annual precipitation and temperature were found significantly linked to the functional structure of microbial communities in a soil transplant experiment designed to simulate climate change25. Annual precipitation was a larger contributor to variation in bacterial community structure than soil pH in the maize cropping systems. This might be attributed to the large variability of precipitation among the sampling sites, which were across a large spatial scale and covered large landmass and various climate regions in China. Precipitation could change soil moisture, and sudden change in moisture is stressful to microbes, as they must expend energy to regulate osmotic pressure to their microenvironment26. Additionally, annual precipitation could compensate for water tables and affect redox condition seasonality27. In addition, climate factors can change soil geochemistry and thereby impact microbial community structure. For example, a national soil survey of Scotland revealed that soil pH and precipitation altered the diversity of ammonia oxidizer communities and their capacity for nitrification11. Moreover, the combination of warming and decreased precipitation altered bacterial community structure in a Tibetan plateau alpine grassland after a 1-year manipulation28.
The above findings suggest that precipitation is a major factor impacting bacterial community assembly in soils; this is supported by our observations in the current study. A few dominant phyla were significantly correlated with mean annual precipitation in the maize cropping systems. With increasing annual precipitation, Proteobacteria increased, while Gemmatimonadetes, and Nitrospirae decreased in maize soils. Similarly, altered precipitation was found to specifically impact Gammaproteobacteria in alpine grassland28. Another study found that Gemmatimonadetes was inversely correlated with soil moisture29.
An important issue in biogeography is estimating whether spatial distance creates genetic variation5. The DDR estimates the variation in beta-diversity across spatial scales30,31. In the present study, the variation in soil bacterial community decreased linearly with increasing geographic distance, indicating the spatial structure of bacterial communities in maize soils. This result could be supported by other studies demonstrating the robust DDRs of microbial communities in agricultural fields32,33.
Various spatial scales could result in a difference in the distance-decay slope between habitats due to the dependence of distance-decay on the spatial scale34,35, which is known to affect the assembly of soil bacterial communities in wheat fields33. Here we found that the distance-decay slope (w = −0.043) in maize soils with an interval of 4129.6 km was steeper than in dryland habitats of northern China across a 4000 km transect35, including alpine grassland (w = −0.041), desert (w = −0.017), desert grassland (w = −0.014), and typical grassland (w = −0.017). The slopes of these relationships across habitats can differ, reflecting varying rates of species turnover in their habitats35. In particular, agricultural fields are typical human-managed terrestrial ecosystems, resulting in distinct microbial community assembly patterns compared to natural ecosystems36. Agricultural soils can form unique, ephemeral habitats across local sites during long-term tillage37, thus generating more spatially structured microbial communities across large scales. Dispersal limitations, which can be attributed to geographic distances, reflect a stochastic process that influences the beta diversity and biogeographic patterns of microbes5. This is in accordance with previous findings in diverse habitats that microbial distributions are not only driven by deterministic process (i.e., environmental heterogeneity), but can also be governed by stochastic process (i.e., dispersal limitations)6,7.
Furthermore, a number of bacterial phyla showed significant DDRs with differing slopes in the maize soils, indicating their distinct turnover rates along the spatial gradient. The dominant phyla Proteobacteria, Actinobacteria, and Acidobacteria had lower slopes and stronger dispersal capabilities, while the less abundant phyla Planctomycetes and Verrucomicrobia showed lower dispersal capabilities. Dispersal capabilities may be linked to phylum abundance. Abundant bacteria are readily dispersed, as many individual cells can potentially be involved in a dispersal event; rare bacteria with lower abundances should show lower dispersal rates than abundant taxa38. Verreydt, et al.39 found that the dispersal capabilities of bacterial groups varied greatly in aquatic habitats, similar to our observations in maize soils.
In particular, we found that Proteobacteria and Gemmatimonadetes were correlated to both geographic distance and environmental factors, although no autocorrelations were found between geographic distance and edaphic factors. Thus, the change in the relative abundances of particular microbes may be ascribed to both dispersal limitations and environmental heterogeneity. Stochastic processes generate random variation in the relative abundances of species (i.e., dispersal limitation and ecological drift) and create patchiness in community composition40. Deterministic processes indicate that environmental selection including abiotic and biotic factors could determine the community assembly41. The above results indicate the combined influence of deterministic and stochastic processes on the bacterial community assembly in maize cropping systems.
Network analysis was used to explore the bacterial co-occurrence patterns and provide insights into bacterial interactions in soils from maize cropping systems. When compared with a random network, the non-random co-occurrence of the bacterial communities was observed across the maize soils; this indicates the role of deterministic processes in bacterial community assembly of agro-ecosystems7. In this study, the core microbiome was broadly distributed and accounted for a high fraction of community beta diversity but a low proportion of total OTUs in the maize soils. According to the node-level (betweenness centrality) and network-level topological features, we found that members of the core microbiome were more often located in central ecological positions than other taxa. A more connected and complex sub-network was therefore generated for the core microbiome. Betweenness centrality scores can be used to determine the most influential taxa (e.g., keystone species) that are responsible for maintaining connectivity within the ecological network42. Thus, the core microbiome might play a vital role in maintaining the complex connections between microbes in agro-ecosystems.
In a co-occurrence network analysis, the topological features of nodes are used to determine the potential importance of microorganisms, such as keystone species43,44,45. Different with core taxa, keystone species were determined via network analysis. Based on betweenness centrality scores, Aeromicrobium, Friedmanniella, Saccharothrix, Lamia, Rhodococcus, Skermanella, and Pedobacter were considered keystone taxa in the maize soils. Aeromicrobium and Rhodococcus are characteristic of herbicide metabolism46. Friedmanniella has been detected in lignocellulose degradation of composted agricultural waste47. Saccharothrix possesses antifungal activity48. Pedobacter has been isolated from a herbicide-contaminated soil49. These keystone taxa may play versatile roles in soil ecological processes in agro-ecosystems.
Sources of uncertainties should be noted when interpreting the results of this study. Firstly, number of samples is small and there are no duplicates at each site, which may affect our results. In the further work, three composite samples per site would be taken into account. Second, although we sampled at the same time, we failed to consider the specific management system of each site, such as the variety of corn, the amount of fertilizer applied and the farming method. Therefore, in addition to natural rainfall, the source of water is then agricultural irrigation, but our study did not include artificial water supply due to large-scale sampling, which may still influence the results.
In conclusion, this is a detailed study of soil bacterial communities in maize cropping systems across a continental scale in China. We determined the major environmental factors correlating with bacterial community diversity and explored the bacterial co-occurrence patterns in 21 maize soils across China. Mean annual precipitation and soil pH were the main factors that shaped the bacterial communities. Different spatial turnover rates of microbes suggest distinct dispersal capabilities of different bacterial groups. The central ecological role of the core microbiome suggests its vital roles in promoting soil ecological processes and maintaining the complex bacterial network. The non-random co-occurrence patterns and identification of keystone taxa provide new insights into microbial community assembly in agro-ecosystems. Furthermore, microbial taxonomic and functional data across temporal scales should be integrated to better characterize the patterns of microbial biogeography and dynamics in agro-ecosystems. Particularly, the investigation the specific distribution of the core microbiomes across different crops agro-ecosystems might improve our understanding of nutrient management and crop health in agro-ecosystems, which should be further explored.
Source: Ecology - nature.com


