Silicon sorption to goethite surfaces
In a first experiment, we examined Si sorption by comparing the relationship between dissolved Si concentrations in the equilibrium solutions after sorption and amounts of Si sorbed. Here, the dissolved Si concentrations varied between 0 and 819 µM, covering the common range of soil solution concentrations1. The relationship between Si in equilibrium solutions and amounts of Si sorbed (Si ‘loading’ levels) showed a logarithmic shape, i.e., the increases in Si loadings diminished at higher dissolved Si concentrations, yet, no clear plateau level indicating maximum surface coverage appeared (Fig. 1). The maximum Si surface loading (achieved at equilibrium concentrations of 819 µM Si) was 79 µmol Si g−1 or 2.2 µmol Si m−2. In contrast to our findings, other authors found steep increases in Si surface loadings at higher dissolved Si concentrations, which was explained by Si polymerization at Fe oxide surfaces (see data compilation and discussion in Song et al.25). In the study system at pH 4, the leveling-off of surface loadings at higher Si concentrations rather suggests limited availability of sorption sites for Si, which hints at Si sorption primarily via formation of Fe–O–Si bonds. It should be noted that presence of H2CO3 in solutions may have reduced Si sorption at pH 426. In case of such an interference, it should be most pronounced at small Si concentrations since the H2CO3 concentration does not depend on Si level. Based on equilibrium calculations (Visual MINTEQ; ver. 3.1; KTH, SEED, Stockholm, Sweden), we estimated that the H2CO3 concentration in solutions were ~ 12 µM, which is in the range of the lower Si concentrations used (i.e., 4–72 µM Si for the three initial solutions with the lowest Si concentrations). Yet, the finding that the sorption envelope showed no deviation from the typical L shape at smaller Si concentrations (Fig. 1), suggests no or negligible repression of Si sorption at smaller concentrations.
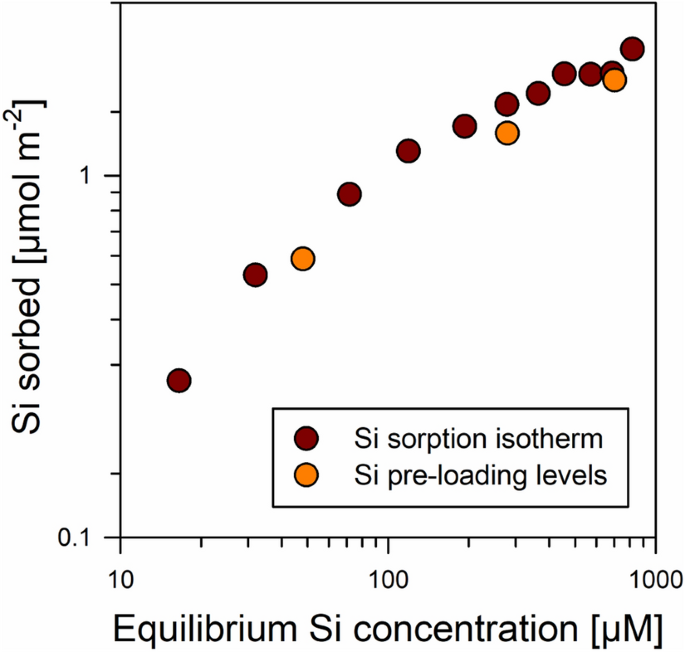
Relationship between equilibrium Si concentrations in solution and amounts of Si sorbed at the goethite surfaces for different experiments (Si sorption test and Si pre-loading of goethite for the experiments with forest floor solutions). Error bars (X- and Y-axis), denoting the standard deviation of replicates (n = 3), are smaller than the symbols. Data were plotted using SigmaPlot 11.0 from Systat Software, San Jose, CA.
Pre-loading of the goethite with silicon
On basis of the data presented above, we selected the levels of Si pre-loading for the subsequent experiments with natural forest floor solutions. The results on Si sorption during the pre-loading procedure matched the results of the prior sorption experiment (Fig. 1), indicating good experimental reproducibility. Rinsing the goethite for removal of residual salts removed weakly bound Si from the surfaces; the losses averaged 5% of the initial surface-bound Si. The final Si loading levels of the samples (after sorption and washing) were 20, 44, and 60 µmol Si g−1 or 0.5, 1.1, and 1.7 µmol Si m−2, which represented 25%, 56%, and 76% of the maximum Si surface loading achieved in the prior sorption experiment. No higher loading levels were used to avoid Si polymerization at the surfaces, and thus, other Si binding types being involved in the sorption competition. The Si pre-loading levels of samples are denoted in the following with ‘low’, ‘medium’, and ‘high’.
The pre-loading of the goethite with Si had no clear effect on the SSA, which averaged 38.4 m2 g−1 for all used goethite samples, including the one without Si loading (Table 1). This value was within the range of values reported for goethites27,28. Similarly, we found no large differences in the ζ potential (pH 4) between the Si pre-loading levels, which equaled on average 40.8 mV (Table 1). Hence, surface area and charge of the different Si pre-loading levels were no factors in the sorption experiments with natural forest floor solutions.
The Si/Fe elemental ratios calculated on basis of XPS data were 3.9∙10−2 ± 1.4∙10–2, 6.0∙10–2 ± 1.2∙10–2, and 6.6∙10–2 ± 4.1∙10–3 for the ‘low’, ‘medium’, and ‘high’ Si pre-loading levels. According to Swedlund et al. (2011)29, shifts of the XPS Si 2s signal by up to 1 eV towards higher binding energies are indicative of increasing contribution of Si–O–Si bonds, and thus, increasing surface polymerization. We did not find such shifts in binding energy with increasing Si pre-loading (Fig. 2). Instead, the binding energies were in the range reported for predominately Fe–O–Si bonds29. In particular, signal maxima were at 152.3 ± 0.4 eV, 152.2 ± 0.3 eV, and 152.0 ± 0.2 eV for the ‘low’, ‘medium’, and ‘high’ Si pre-loading levels (average values and standard deviation of three analysis positions). The XPS results thus support the data from the Si sorption experiment, suggesting that Si at the goethite surface was primarily coordinated in Fe–O–Si bonds, and that no Si polymers were present. The lack of detectable Si polymerization at the surfaces (i.e., XPS data and Si sorption curve) may be explained by the short reaction time of 24 h. While sorption of monomeric Si to surficial Fe atoms is relatively fast and typically reaches its maxima after minutes to a few hours6, surface polymerization is slower; it starts later and can continue over long periods of time6,11. In studies on the sorption of Si to hematite, even after 210 days, no equilibrium was reached due to progressing surface polymerization11. Also in 60-days laboratory experiments with soil material, Si sorption increased until the end of the observation period30. Hence, our data apply to sorption phenomena in relatively fast draining soil material. Due to continuous percolation, contact times between soil solution and the solid phases are often short in many topsoils under humid climate. For example, in an acidic forest soil (Dystric Cambisol) in Northern Germany, water fluxes at 10 cm soil depth mirrored well the precipitation events, and even small events resulted in percolating water; it was estimated that water fluxes at 10 cm soil depth represented 70% of the throughfall31. An additional factor of Si polymerization (which was not assessed herein) may be the morphology of goethite samples, which determines relative distribution of surface types; more particularly, the work of Song et al.25 suggests that (021) crystal faces of goethite promote polymerization, whereas (110) faces do not allow polymerization.
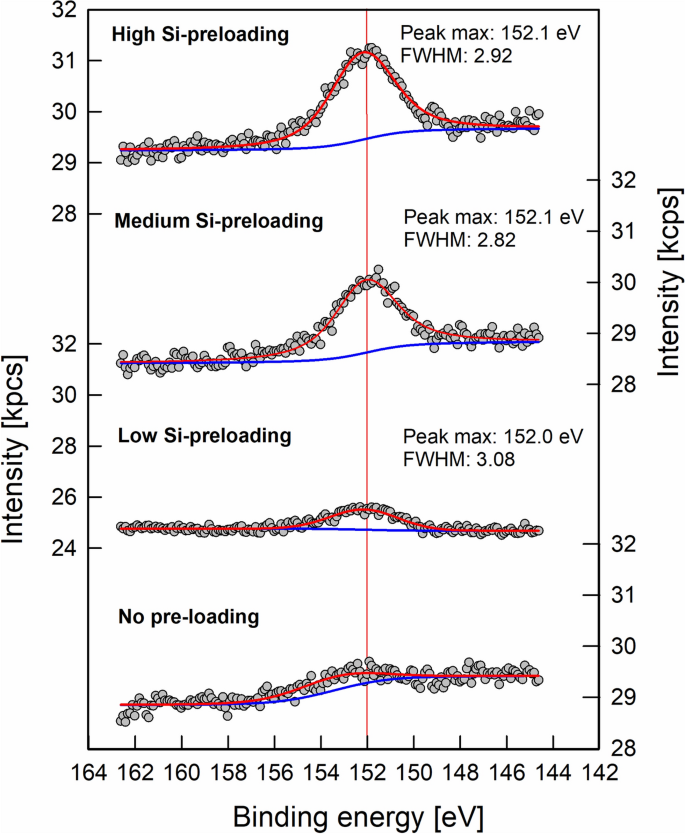
Examples of XPS spectra of the Si 2s region for goethite with variable Si pre-loadings prior to the sorption experiments with forest floor solutions. The vertical red line at 152.0 eV highlights that there were no differences in peak position between pre-loading levels, thus indicating the prevalence of monomeric Si. Data were plotted using SigmaPlot 11.0 from Systat Software, San Jose, CA.
Initial properties of the natural forest floor solutions
The pH value was slightly higher in the Bad Brückenau than in the Mitterfels solution (4.7 vs. 4.1; Table 2), and this difference might have contributed to the differences in sorption processes discussed below. It has been demonstrated for the pH range studied here that the amounts of Si sorbing to Fe oxide surfaces increase with increasing pH3, while the sorption of DOM typically decreases32.
The Bad Brückenau solution had higher initial DOC and dissolved Si concentrations (Table 2), and the initial molar C/Si ratios in the solutions were 16.5 (Bad Brückenau) and 36.6 (Mitterfels). Initial SUVA280 values were similar for the two solutions (Table 1), suggesting similar relative contributions of aromatic moieties to DOM. We determined the aromaticity of DOM because aromatic components of forest floor solutions have a high affinity to bind to Fe oxide surfaces33. Given the higher molar C/Si ratio and similar SUVA280 values, we expected that the DOM of the Mitterfels solution is more competitive against Si for sorption sites than those of the Bad Brückenau solution.
The total P and phosphate-P concentrations were higher in the Mitterfels than in the Bad Brückenau solution (Table 2). In both solutions, the initial phosphate-P concentrations made only about 3% of the total P concentrations, suggesting most P to be present in organic form. Moreover, as phosphate-P concentrations made less than 1% of the dissolved Si concentrations, it played only a minor role as a competitor for sorption sites in the present study.
Sorption of forest floor solution constituents to goethite without Si pre-loading
The DOC concentrations decreased with increasing addition of goethite for both forest floor solutions (Fig. 3). The maximum decrease was by 93% for the Bad Brückenau solution and by 88% for the Mitterfels solution, re-confirming the well-established strong DOC sorption by goethite34,35. At goethite additions ≥ 6 g l−1 for the Bad Brückenau solution and ≥ 2 g l−1 for the Mitterfels solution, no additional DOC was adsorbed. This suggests that the portions of organic compounds in the solutions capable to bind were limited and already completely sorbed once a certain level of sorption sites was offered. In turn, the C loading of the goethite surfaces decreased with increasing goethite addition, i.e., when increasing amounts of sorption sites offered (Fig. 4). Maximum values were 1.0 mmol g−1 (0.03 mmol m−2) for the Bad Brückenau solution and 0.8 mmol g−1 (0.02 mmol m−2) for the Mitterfels solution. For the Bad Brückenau solution, the C loadings were similarly high for goethite additions of 0.5 and 2 g l−1, suggesting that the surfaces were ‘saturated’ with organic matter, i.e., all sorption sites available for potentially sorbing DOM components were occupied, leaving compounds with weaker sorption affinities in solution. The C saturation level observed for the Bad Brückenau solution was at the lower end of values reported in studies using a comparable experimental design34,35,36.
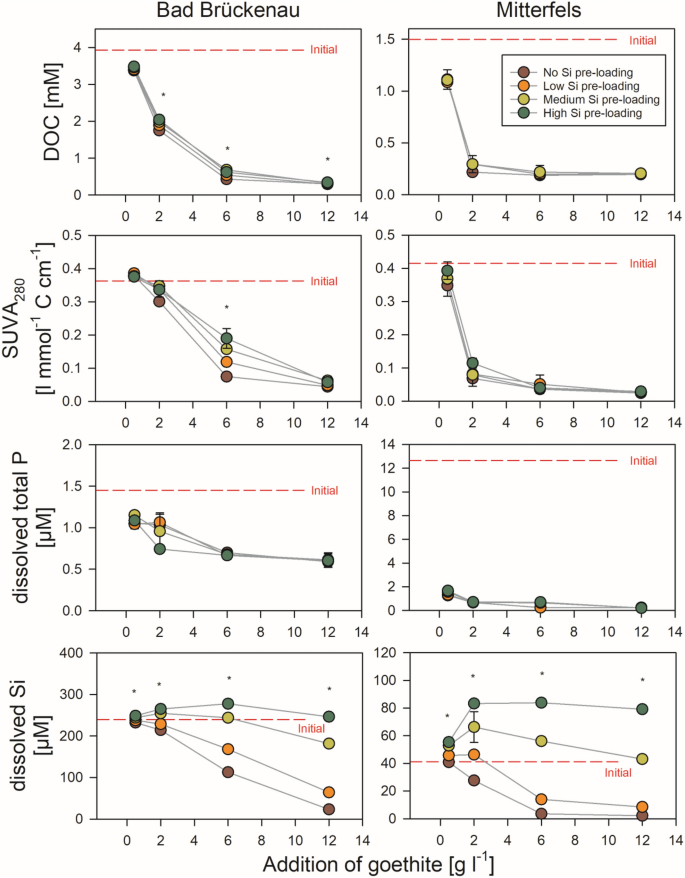
Changes in composition of the two forest floor solutions upon different additions of goethite with and without Si pre-loadings. The error bars indicate the standard deviation of experimental replicates; stars indicate significant differences between treatments (p < 0.05; ANOVA on ranks), i.e. at least two treatments differ significantly from each other. SUVA280 indicates the specific UV absorbance measured at 280 nm and normalized to DOC. Data were plotted using SigmaPlot 11.0 from Systat Software, San Jose, CA.
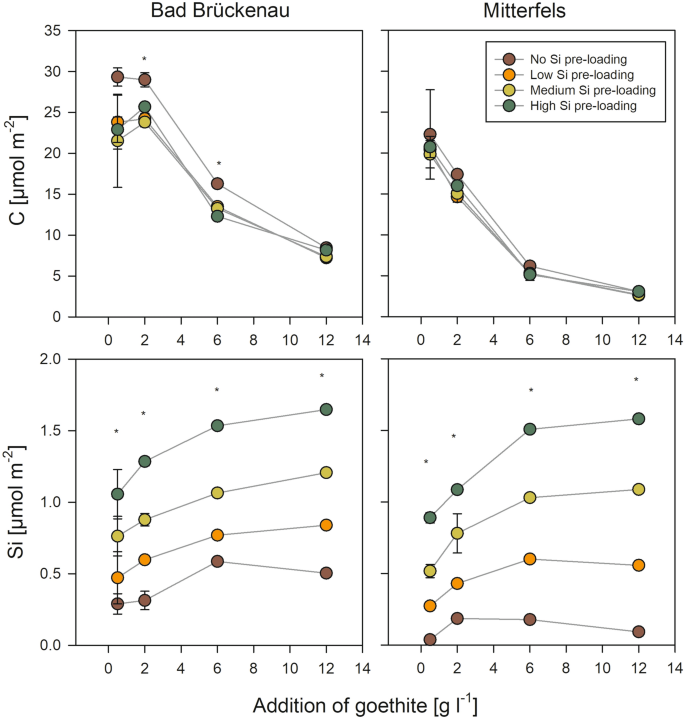
Amount of organic C and Si at goethite surfaces after interaction with the two forest floor solutions upon different additions of goethite with and without Si pre-loadings. The error bars indicate the standard deviation for experimental replicates; stars indicate significant differences between treatments (p < 0.05; ANOVA on ranks), i.e. at least two treatments differ significantly from each other. Data were plotted using SigmaPlot 11.0 from Systat Software, San Jose, CA.
Decreases in SUVA280 values upon goethite additions indicated selective removal of aromatic components from the forest floor leachates (Fig. 3), which is consistent with literature reports33. The aromaticity of DOM, typically, is higher in case the DOM derives from more decomposed plant litter (as stored, e.g., in lower parts of forest floor layers) than from fresh litter, probably due to the higher contribution of soluble aromatic products of lignin depolymerization released during later stages of litter decomposition37. These oxidized components are rich in carboxyl groups that can form strong inner-sphere complexes at the surfaces of metal oxide phases, particularly at lower pH values (as in our study) when many surface hydroxyl groups at the oxide surfaces are protonated and positively charged36.
Addition of goethite without Si-preloading decreased the concentrations of total P and phosphate in both solutions. Even the smallest addition of goethite caused a decrease in phosphate concentrations to values below detection limit (not shown). This corresponded to the well-known high sorption affinity of phosphate to Fe oxide surfaces under acidic conditions38. The decreases in total P concentrations were up to 59% (Bad Brückenau solution) and 98% (Mitterfels solution) of the initial values (Fig. 3). Especially for the Bad Brückenau solution, the relatively high amount of P remaining in solution demonstrates that some of the P-containing organic compounds can have a low sorption affinity for goethite surfaces even under acidic conditions. This finding agrees well with results from previous sorption experiments and field studies39, 40.
Addition of low amounts of goethite without Si pre-loading (0.5 g l−1) resulted in no or only small decreases in dissolved Si concentrations (< 2%; Fig. 3), which indicates a lower sorption affinity of Si than of other components of the solutions. At higher goethite additions, with more sorption sites being available, considerable decreases in dissolved Si concentrations were found; it decreased by 90% in the Bad Brückenau and by 95% in the Mitterfels solution (Fig. 3). The maximal Si loadings achieved were 21 µmol g−1 or 0.6 µmol m−2 (Bad Brückenau solution) and 7 µmol g−1 or 0.2 µmol m−2 (Mitterfels solution), respectively. The Si loadings showed the opposite trend as the C loadings; they increased with increasing goethite addition (Fig. 4). This also points at a lower sorption affinity of Si than of C, i.e., Si only sorbs to a larger extent when excess sorption sites are available.
Moreover, for goethite additions of up to 2 g l−1 (Mitterfels solution) or 6 g l−1 (Bad Brückenau solution), the molar ratios between DOC and dissolved Si decreased with increasing goethite input (Mitterfels solution: from 27 to 8; Bad Brückenau solution: from 15 to 4). At higher goethite additions, they increased again (Mitterfels solution: from 8 to 92; Bad Bückenau solution: from 4 to 13). This suggests that Si was only sorbed when all strongly sorbing organic matter components were already removed from solution and still some sorption sites were available.
Sorption of forest floor solution constituents to goethite with Si pre-loadings
Effects of pre-loaded Si on changes in DOC (Fig. 3) as well as on C loadings of the goethite surfaces (Fig. 4) were rather small compared to the effects of adding different amounts of goethite. For the Mitterfels solution, no significant differences between Si pre-loading levels were found, and for the Bad Brückenau solution the difference were significant only in few cases. For these cases, our data indicate that the Si pre-loadings reduced the sorption of DOM components. This reduction (as calculated by changes in DOC concentrations) made up to 13% of the amounts of DOC sorbed to goethite without Si pre-loading.
One possible reason for the small effects of the Si pre-loading on the C loadings is the seemingly weak sorption affinity of Si for sorption sites. We found that Si was released into the solutions from goethite with medium and high Si pre-loadings, causing increases in dissolved Si concentrations by up to 31 µM (Bad Brückenau) and 42 µM (Mitterfels; Fig. 5). The portion of initially surface-bound Si released into solution showed a decrease with increasing availability of sorption sites (i.e., with increasing goethite addition; Fig. 5). This finding clearly indicates that Si was displaced from the goethite surface by compounds with a higher sorption affinity, such as phosphate and DOM components. The maximum portions of Si released were 37% (Bad Brückenau solution) and 53% (Mitterfels solution) of the initial Si present at the surfaces. Hence, a large fraction of the pre-sorbed Si was not removed by reaction with the forest floor solutions, and as a consequence, the Si loading at the goethite surfaces after the sorption experiments differed significantly between samples with different initial Si loadings (Fig. 4). These calculations suggest that not all results can be explained by sorption competition, and that additional factors need to be considered for explaining the small effect of Si pre-loadings on the sorption of other forest floor leachate components. One could be that maximum Si coverage used in the experiments was only 76% of the maximum Si surface loading achieved in the prior sorption experiment. An incomplete Si surface coverage of the goethite was also indicated by the small surface Si/Fe ratios determined by XPS. Data obtained in our laboratory within another study showed that the studied goethite binds up to about 90 µmol ortho-phosphate g−1 until the plateau level is reached; this is about 1.5 times the amounts of Si bound at the goethite surfaces at the highest Si pre-loading levels used here. Hence, a considerable part of the other forest floor leachate components might have been bound to sorption sites not occupied by Si, and thus, competition and subsequent displacement of Si become only relevant for the small goethite additions, where available sorption sites were more limited. In addition, the only partial competitive sorption theoretically may hint at differing preferences of Si and organic compounds for certain parts of the goethite surface.
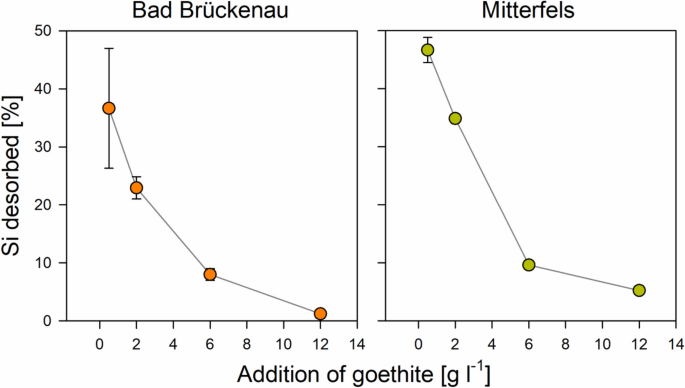
Percentage of Si desorbed from the goethite surfaces with high Si pre-loading due to interactions with the two forest floor solutions as a function of the goethite amounts added (data for other Si-preloading levels are not shown because less pronounced or no desorption occurred); the error bars indicate the standard deviation for experimental replicates. Data were plotted using SigmaPlot 11.0 from Systat Software, San Jose, CA.
Implications
We present for the first time data on competitive interactions between Si and other dissolved components of natural forest floor solutions, such as DOM and phosphate, at goethite surfaces. Overall, our experiments with goethite with and without Si pre-loading suggest that under acidic conditions the competitive strength of Si for sorption sites is low when compared to that of DOM and phosphate (Fig. 6). This implies that in acidic topsoil environments, where input of competitive organic and inorganic components with forest floor leachates is high, retention and accumulation of Si (either from mineral weathering or entering with forest floor leachates) at the surfaces of newly formed hydrous Fe oxides or other similar mineral phases should be comparatively little, while mobility of Si should be high. Nevertheless, we observed significant Si sorption at higher goethite additions, i.e., when more sorption sites were available, a situation that is more typical for subsoils. Hence, sorption competition should be a major factor affecting the partitioning of Si between the dissolved and solid phase within soil profiles. Future work needs to address how this determines major aspects of the biogeochemical Si cycle, including Si phytoavailability, formation of secondary silicate minerals as well as export of Si from soil into the hydrosphere.
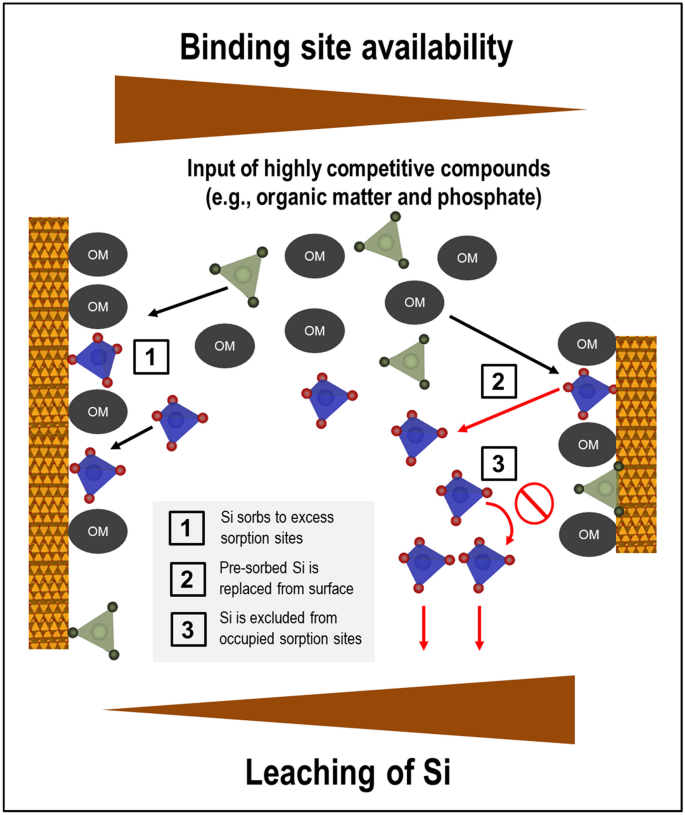
Nature and implications of sorption competition between Si (blue symbols), organic matter (dark grey symbols), and phosphorus (green symbols) at the variable-charge surfaces of pedogenic minerals.
Source: Ecology - nature.com


