Insects
Laboratory-started nests of buff-tailed bumblebee (Bombus terrestris) were obtained from The Research Institute of Fodder Crops (Troubsko, Czech Republic)12. The nests were exposed to colonization by A. sociella L. (Pyralidae, Galleriinae) in the field (Prague area). In June/July, the bumblebee hives were situated in the garden and let parasitize by natural population of A. sociella. In autumn, the infested hives with larvae were transferred to refrigerator and kept 1 month at 5 °C for the larvae to undergo hibernation. After hibernation, the hives were kept in the rearing room (24 °C, 40% relative humidity). Larvae were allowed to develop and pupate in the bumblebee hives. The newly emerged adults were collected and sexed. The sexes were kept separately first in continuous light under ambient laboratory temperature (20–23 °C). Adults (1–2 days old) were transferred to the dark and used for pheromone extraction. Moths (one day post-emergence) for electrophysiological experiments were kept in a refrigerator with the temperature set to 5 °C and in a high-humidity environment (90%) until used.
Sample preparation
Male sex pheromone
The pheromone is produced in wing glands that are located on the basal part of the forewing, analogically as in Eldana saccharina Walker (Lepidoptera, Galleriinae) published by Farine13. Glands of 20 calling males were clipped out, pooled together and soaked in redistilled n-hexane (Merck, 50 μL per gland). Samples were sonicated for 15 min, and extracted for 24 h. The extracts were filtered through purified glass wool, concentrated under argon flow to double the initial concentration, and stored at −20 °C.
Female aphrodisiac pheromone
Virgin females (1–2 days old) were placed individually in cages, and positioned next to caged calling males. Females (20 specimens) that elicited calling songs in males were cooled to −20 °C for 15 min, and their whole bodies (without wings) were pooled and extracted with n-hexane (500 μL n-hexane per female). After 24 h, the female bodies were removed from the extract, the solution was filtered through the glass wool, concentrated to 2 mL under the stream of argon, and the samples were stored at −20 °C.
Analysis of crude extract
Extracts from males and females were analyzed using a Hewlett-Packard 6890 N gas chromatograph (GC) equipped with a polar Varian FactorFOUR VF-23ms column (30 m × 0.25 mm i.d., df = 0.25 µm) and a HP 5973 MS-detector in electron ionization mode (EI). The carrier gas (1 mL min−1) was helium; the sample (1 µL) was injected in the splitless mode, the injector temperature was 250 °C, the transfer line was maintained at 250 °C and the MS source was set to 280 °C. For identification of the alcohol and ketone in crude extracts, the full scan mode was used and the column temperature increased from 50 °C by 10 °C min−1 up to 230 °C and held at 230 °C for 10 minutes. Mass spectra of main extract components are in the Supplementary material (Figs. S1–S3).
Purification of insect extracts
Extracts were purified using liquid chromatography (LC). Prior purification, female extracts were washed with NaHCO3 (sat.aq.) to remove free carboxylic acids. Afterwards, male and female extracts were treated in the same way. In LC purification, pre-conditioned solid phase extraction column (500 mg, SPE, Chromabond) were used. SPE conditioning was done with EtOAc (1.5 mL), 10% EtOAc in pentane (1.5 mL) and pentane (1.5 mL). Then the columns were gradually eluted in steps of 1% increase of ethyl acetate in pentane (0–15%) with each fraction consisted of a volume of 750 µL.
TMPD-one was enriched in fractions 3–6 and TMPD-ol was enriched in fractions 9−11. In cases where the purity was not satisfactory, the combined fractions were subjected to the purification protocol again until no further improvement of purity was obtained. The combined TMPD-one fractions were then reduced to alcohols (described below). After reduction, the TMPD-ol fractions and those containing the reduced TMPD-one were derivatized and analyzed as described below (Fig. 1D).
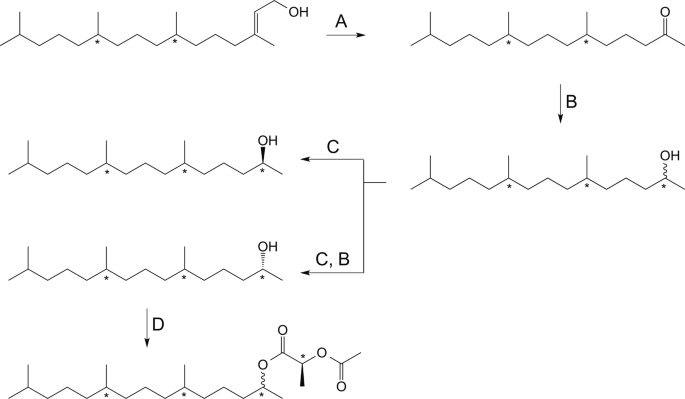
Synthesis of reference compounds and derivatization of reference compounds and natural compounds enriched from insects extracts. (A) NaIO4, RuCl3(cat.), acetonitrile, water, room temperature. (B) Lithium aluminum hydride in diethyl ether, 0 °C 1 h, (C) Candida antarctica lipase B (CAL-B), 4 A molecular sieves, vinyl acetate, heptane, (D) (S)-2-acetoxypropionyl chloride, pyridine, dichloromethane and cyclohexane, 80 °C 1 h then HCl (1 M) and pentane/cyclohexane and drying over Na2SO4 (anhydr.).
Reduction of TMPD-one fraction
The collected ketone fractions were pooled and solvent evaporated under a stream of argon. The purified ketone was diluted in dry diethyl ether (1 mL) and 0.2 mL was reduced to alcohol with lithiumaluminumhydride in diethyl ether (1 M, 50 µL) at 0 °C for 1 hour (Fig. 1B). The reaction was quenched by addition of HCl (2 M, 100 µL) and H2O (100 µL). Phases were separated and the aqueous layer was extracted with Et2O (3 × 200 µL), the combined organic layers was washed with HCl (2 M, 100 µL) and brine (100 µL) and dried over Na2SO4 (anhydr.) in a Pasteur pipette. The solvent was evaporated under a stream of argon. The sample was diluted with pentane (50 µL) and purified with the same SPE purification method as the crude extracts, prior to derivatization.
Derivatization
To determine the stereochemistry of TMPD-ol enriched from female extract and TMPD-one enriched from male extract of A. sociella, both the pooled alcohol fraction and the reduced ketone fraction were derivatized with (S)−2-acetoxypropionyl chloride (SigmaAldrich) according to Bång14. Pooled extracted TMPD-ol and reduced TMPD-one were concentrated by means of evaporating solvent under a stream of argon. Dry cyclohexane (50 µL) was added to the vial containing the purified extract and subsequently was 1% pyridine in cyclohexane (65 µL) and 1% (S)-2-acetoxypropionyl chloride in dichloromethane (100 µL) added, the resulting mixture was heated at 80 °C for 30–60 minutes under argon atmosphere (Fig. 1D). A needle was inserted into the septum of the vial and the solvent was allowed to evaporate before allowing the vial to reach room temperature. HCl (1 M, 200 µL) and pentane or cyclohexane (1 mL) was added, the organic layer was subsequently dried over Na2SO4 (anhydr.) in a Pasteur pipette. Solvent was either reduced to approximately 50 μL and the sample was analyzed as described below or the solvent was evaporated completely and sample dissolved in cyclohexane before analysis.
Stereochemical analysis
The derivatized TMPD-ol was analyzed using GC/MS as described above for the crude extracts. The temperature program was adjusted, starting at 50 °C, increased to 110 °C at a rate of 10 °C min−1, further from 110 °C by 0.01 °C min−1 up to 115 °C, and from 115 °C by 10 °C min−1 up to 230 °C, and held at 230 °C for 10 minutes. Selected ion monitoring (SIM) (m/z 105, 115, 133, 210, 252) was used for identification of the (S)-2-acetoxypropionyl derivatives10.
Preparation of reference compounds
(2R/S,6R/S,10R/S)–6,10,14-Trimethylpentadecan-2-ol (2E,7 R/S,11 R/S)-(3,7,11,15)-Tetramethyl-2-hexadecen-1-ol ((2E,7R/S,11R/S)–phytol) (SigmaAldrich) was oxidized at room temperature by sodium periodate and catalytic amount of ruthenium (III) chloride (Fig. 1A), the obtained ketone was reduced with LiAlH4 in diethyl ether resulting in a mixture of eight isomers of TMPD-ol (Fig. 1B) according to the supplemental material of Nieberding et al.9. The synthetic mixture was derivatized and analyzed according to the same method as the purified extracts.
(2S,6R,10R)–6,10,14-Trimethylpentadecan-2-ol and (2R,6R,10R)–6,10,14-trimethylpentadecan-2-ol (2E,7R,11R)-Phytol (TCI America) was treated as described above, resulting in a mixture of (2R,6R,10R)- and (2S,6R,10R)-TMPD-ol (Fig. 1B). The isomeric mixture was subjected to enzymatic resolution employing Candida antarctica lipase-B (CALB, Roche Diagnostics batch 90750729) as catalyst and vinylacetate (SigmaAldrich) as acyl-donor (Fig. 1C). With this method the (2 R)-alcohol is acetylated and therefore the two isomers are easily separated by means of liquid chromatography. The obtained (2 R)-acetate was reduced with litiumaluminum hydride in diethyl ether (Fig. 1B). A diastereomeric purity of 99.8% for (−)-(2R,6R,10R)-TMPD-ol and 97.8% diastereomeric purity of (+)-(2S,6R,10R)-TMPD-ol was obtained as described in the supplemental material of Nieberding et al.15. The stereoisomerically pure isomers were derivatized and analyzed according to the same method as the purified extracts.
Electroantennography (EAG)
Detailed description of EAG measurements on A. sociella is available in Kalinová et al.5. Briefly, isolated antennae, glass Ag/AgCl electrodes and Syntech EAG recording and stimulus delivery system were used. Male and female antennal responses to stimulation by 10, 100, and 250 ng of racemic mixtures and pure enantiomers of TMPD-one and TMPD-ol were investigated. Tested compounds were diluted in n-hexane. Aliquots of 10 μL of respective solutions were loaded onto the filter paper discs within Pasteur pipette odor cartridges. During stimulation, 0.5 mL of humidized air was flown through loaded odor cartridge and injected into the air stream directed onto the antennal preparation. For each sex and stimulus type, 6 replicates were performed.
The antennal responses from each series of stimulations were normalized to the stimulations by 10 ng of 1-hexenol using the Syntech software and expressed as percents of 1-hexenol responses. For statistical evaluation, the data were subjected to logarithmic transformation to correct for the heteroscedasticity inherent to EAG data. The resulting datasets complied with the assumption of equal variances (tested using Levene’s test) and the means were compared using ANOVA followed by Least Squares Differences post-hoc multiple comparison test, performed in Statistica 8.0.
Dedication
Dedicated to the memory of Prof. Kenji Mori and his significant contribution to the field of chemical ecology.
Source: Ecology - nature.com


