Jennifer M. Sunday
Contact
Search for this author in:
Projections of the effects of climate change on multiple species are often made by estimating the change predicted for a single future time point; for example, by asking how the geographical distributions of multiple species will differ in 2100 from those today1. However, this approach does not capture the pace, timing or possible synchrony of biodiversity changes across time. Acute synchronous impacts can potentially be more damaging to a system than those spread over time, in terms of both human adaptation to biodiversity losses and ecosystem resilience. Writing in Nature, Trisos et al.2 report an approach for predicting how climate change will affect future biodiversity patterns.
The authors estimated the timing and synchrony of climate impacts on organisms globally by asking when species in a given region will be exposed to temperatures outside their normal global experience (by considering projected future temperatures due to climate change). They did this by compiling geographical-range maps for approximately 30,000 species, including birds, mammals, reptiles, amphibians, fishes, marine invertebrates, corals and seagrasses, and using temperature-projection models to identify the warmest average annual temperature experienced between 1850 and 2005 by each species within its range. Dividing Earth into grid cells of 100 square kilometres and using predicted climate information, the authors determined when each species would experience annual average temperatures above its historical annual average, encountered anywhere in its range, for an extended period. The result provides an estimate of when a species will be exposed to unprecedentedly high temperatures.
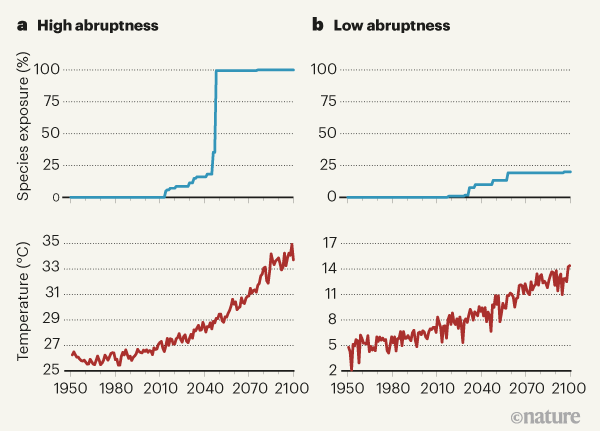
Trisos and co-workers’ approach builds on ‘time of emergence’, a concept used when analysing climate change. Time of emergence describes the time at which a climate variable, such as temperature, emerges beyond the historical values of variation observed for a particular location — in other words, when the average value of the measurement of interest becomes more extreme than the previously encountered natural variability. Trisos et al. offer innovation in applying this concept to the realm of biodiversity. First, rather than considering the variation experienced at just one location, they considered the full breadth of variation experienced across each species’ geographical range, defining an organism as being ‘exposed’ in a specific grid cell only after it has experienced temperatures above its range-wide maximum (and with annual temperatures remaining above this value for a minimum of five years). Second, because the authors considered multiple species in an assemblage (the group of species present in a given grid cell), it was possible to assess the relative timing of exposure in a graphical format that the authors call a horizon profile (Fig. 1). This enables the synchrony in the timing of exposure events for the species in a region to be quantified and easily visualized.
The authors’ results predict that the greatest levels of exposure will occur at latitudes nearer the Equator, and, most notably, that there will be high synchrony in the timing of exposure between species in the same grid cell, for grid cells both on land and in the ocean. Trisos et al. find that most species in a given cell will usually become exposed to unprecedentedly high temperatures within the same decade. If this exposure results in local extinction, it suggests the following disturbing scenario. We might initially see a small trickle of species being lost from an assemblage, but this will be followed by an abrupt loss of most species in the assemblage within the same decade.
What mechanism might explain this predicted pattern? The abruptness in exposures predicted by Trisos and colleagues is not due to any particular abruptness in the timing of climate change itself — although similar predictions of abrupt ecological change have been based on the additive effects of gradual climate change with abrupt natural climate variability, including weather3. Instead, it seems to be attributable to the similarity of the thermal niches occupied by the species in each grid cell. Trisos and colleagues find that more than half of the species in a given cell (and almost 90% in most marine assemblages) tend to have geographical ranges that encompass similarly warm temperatures, such that they would all face exposure at around the same time.
Such a striking pattern of shared thermal niches within assemblages has been observed before, in a global analysis of marine fishes and invertebrates4. In that study, species’ thermal niches were found not to change gradually with latitude, but instead to have distinct transition points, indicating that species belong to what are termed thermal guilds4. These shared thermal niches could be due to physical boundaries or ecological interactions that restrict the ranges — and temperatures experienced — of multiple species similarly. Or this phenomenon might be the result of a low rate of evolution in the range of temperatures across which the species can fundamentally persist, leading to the maintenance of thermal guilds.
When does this abrupt exposure happen? It is predicted that it will occur at different times for grid cells around the world, from some predicted to be occurring already in the ocean, to others occurring towards the end of the projected time range, in 2100. That the timing is different across grid cells is a good thing, because at least all of the assemblages aren’t predicted to experience abrupt losses at the same time. But, notably, the timing of exposure does not correlate with the timing of climate-change emergence in temperature, suggesting that the latter metric might be a poor predictor of major biodiversity change within a given grid cell.
Trying to project the timing of biodiversity shifts is a noble objective that will surely help us to develop management systems and anticipate crises. Although Trisos et al. provide an initial approach that offers useful insights, further studies should attempt to validate and qualify these predictions. For example, Trisos and colleagues used temperatures outside species’ current thermal niches to define climate exposure, but we don’t know what will really occur when species experience such temperatures — many can certainly tolerate temperatures beyond those found in their current ranges5,6. The timing of exposure to truly limiting environments might turn out to be more diverse across species than currently predicted by Trisos et al. if variation in species’ fundamental climatic niches (the range of temperatures and other climate variables across which an organism can survive) is considered. It will also be useful to consider the flip side of the range-shift issue: the timing and abruptness with which new species enter an assemblage as a result of range extensions arising from climate change.
Most crucially, as climate change progresses, we should be able to test and refine projections such as these using real-time observations. Where are biodiversity changes already occurring abruptly? The need for systematic global biodiversity monitoring has never been stronger.
References
- 1.
Warren, R., Price, J., Graham, E., Forstenhaeusler, N. & VanDerWal, J. Science 360, 791–795 (2018).
- 2.
Trisos, C. H., Merow, C. & Pigot, A. L. Nature https://doi.org/10.1038/s41586-020-2189-9 (2020).
- 3.
Harris, R. M. B. et al. Nature Clim. Change 8, 579–587 (2018).
- 4.
Stuart-Smith, R. D., Edgar, G. J., Barrett, N. S., Kininmonth, S. J. & Bates, A. E. Nature 528, 88–92 (2015).
- 5.
Sunday, J. M., Bates, A. E. & Dulvy, N. K. Nature Clim. Change 2, 686–690 (2012).
- 6.
Early, R. & Sax, D. F. Glob. Ecol. Biogeogr. 23, 1356–1365 (2014).
- 7.
van Vuuren, D. P. et al. Clim. Change 109, 5–31 (2011).
Source: Ecology - nature.com