Fonseca, P. & Revez, M. A. Temperature dependence of cicada songs (Homoptera, Cicadoidea). Journal of comparative Physiology A 187, 971–976 (2002).
Moriyama, M. & Numata, H. Diapause and prolonged development in the embryo and their ecological significance in two cicadas, Cryptotympana facialis and Graptopsaltria nigrofuscata. Journal of insect physiology 54, 1487–1494 (2008).
Toolson, E. C. Comparative thermal physiological ecology of syntopic populations of Cacama valvata and Tibicen bifidus (Homoptera: Cicadidae): modeling fitness consequences of temperature variation. American Zoologist 38, 568–582 (1998).
Sanborn, A. Cicada thermoregulation (Hemiptera, Cicadoidea). (na, 2002).
Sanborn, A. F. & Maté, S. Thermoregulation and the effect of body temperature on call temporal parameters in the cicada Diceroprocta olympusa (Homoptera: Cicadidae). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 125, 141–148 (2000).
Sato, Y. & Sato, S. Spring temperature predicts the long-term molting phenology of two cicadas, Cryptotympana facialis and Graptopsaltria nigrofuscata (Hemiptera: Cicadidae). Annals of the Entomological Society of America 108, 494–500, https://doi.org/10.1093/aesa/sav036 (2015).
Battisti, A. et al. Expansion of geographic range in the pine processionary moth caused by increased winter temperatures. Ecological applications 15, 2084–2096 (2005).
Dale, A. G. & Frank, S. D. The effects of urban warming on herbivore abundance and street tree condition. PloS one 9, e102996 (2014).
Laws, A. N. & Belovsky, G. E. How will species respond to climate change? Examining the effects of temperature and population density on an herbivorous insect. Environmental Entomology 39, 312–319 (2010).
Rueda, L., Patel, K., Axtell, R. & Stinner, R. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). Journal of medical entomology 27, 892–898 (1990).
Heath, J. E. Temperature responses of the periodical “17-year” cicada, Magicicada cassini (Homoptera, Cicadidae). American Midland Naturalist, 64–76 (1967).
Oke, T. R. City size and the urban heat island. Atmospheric Environment (1967) 7, 769–779 (1973).
Nowak, D. J. & Dwyer, J. F. In Handbook of urban and community forestry in the Northeast 11–25 (Springer, 2000).
Oke, T. In Wind climate in cities (eds. Cermark, J. E., Davenport, A. G., Plate, E. J. & Viegas, D. X.) 81–107 (Springer, 1995).
Hart, M. A. & Sailor, D. J. Quantifying the influence of land-use and surface characteristics on spatial variability in the urban heat island. Theoretical and applied climatology 95, 397–406 (2009).
Santamouris, M. Heat island research in Europe: the state of the art. Advances in building energy research 1, 123–150 (2007).
Ryu, Y.-H. & Baik, J.-J. Quantitative analysis of factors contributing to urban heat island intensity. Journal of Applied Meteorology and Climatology 51, 842–854 (2012).
Agrawal, M. In Urban Ecology 603–607 (Springer, 1998).
Stringer, L. D., Stephens, A. E., Suckling, D. M. & Charles, J. G. Ant dominance in urban areas. Urban Ecosystems 12, 503–514 (2009).
Willigalla, C. & Fartmann, T. Patterns in the diversity of dragonflies (Odonata) in cities across Central Europe. European Journal of Entomology 109, 235–245 (2012).
White, J. A. & Lloyd, M. Growth rates of 17 and 13-year periodical cicadas. American Midland Naturalist, 127–143 (1975).
Koyama, T. et al. Geographic body size variation in the periodical cicadas Magicicada: implications for life cycle divergence and local adaptation. Journal of evolutionary biology 28, 1270–1277 (2015).
Karban, R. Evolution of prolonged development: a life table analysis for periodical cicadas. The American Naturalist 150, 446–461 (1997).
Karban, R. Sexual selection, body size and sex-related mortality in the cicada Magicicada cassini. American Midland Naturalist, 324–330 (1983).
Smith, D. M., Kelly, J. F. & Finch, D. M. Cicada emergence in southwestern riparian forest: influences of wildfire and vegetation composition. Ecological Applications 16, 1608–1618 (2006).
Saisho, Y. Mathematical observations on the relation between eclosion periods and the copulation rate of cicadas. Mathematical biosciences and engineering: MBE 7, 443–453 (2010).
Hamblin, A. L., Youngsteadt, E., López-Uribe, M. M. & Frank, S. D. Physiological thermal limits predict differential responses of bees to urban heat-island effects. Biology letters 13, 20170125 (2017).
Schowalter, T. D. Insect ecology: an ecosystem approach. (Academic Press, 2016).
Diamond, S. E., Chick, L. D., Perez, A., Strickler, S. A. & Martin, R. A. Evolution of thermal tolerance and its fitness consequences: parallel and non-parallel responses to urban heat islands across three cities. Proc. R. Soc. B 285, 20180036 (2018).
Piano, E. et al. Urbanization drives community shifts towards thermophilic and dispersive species at local and landscape scales. Global change biology (2017).
Diamond, S. E. et al. A physiological trait-based approach to predicting the responses of species to experimental climate warming. Ecology 93, 2305–2312 (2012).
Stuble, K. L. et al. Foraging by forest ants under experimental climatic warming: a test at two sites. Ecology and evolution 3, 482–491 (2013).
Angilletta, M. J. et al. Urban physiology: city ants possess high heat tolerance. PLoS One 2, e258 (2007).
Diamond, S. E., Chick, L., Perez, A., Strickler, S. A. & Martin, R. A. Rapid evolution of ant thermal tolerance across an urban-rural temperature cline. Biological Journal of the Linnean Society 121, 248–257 (2017).
Lee, Y. J. Revised synonymic list of Cicadidae (Insecta: Hemiptera) from the Korean Peninsula, with the description of a new species and some taxonomic remarks. Proceedings of the Biological Society of Washington 121, 445–467 (2008).
Kim, T. E., Oh, S.-Y., Chang, E. & Jang, Y. Host availability hypothesis: complex interactions with abiotic factors and predators may best explain population densities of cicada species. Animal Cells and Systems 18, 143–153 (2014).
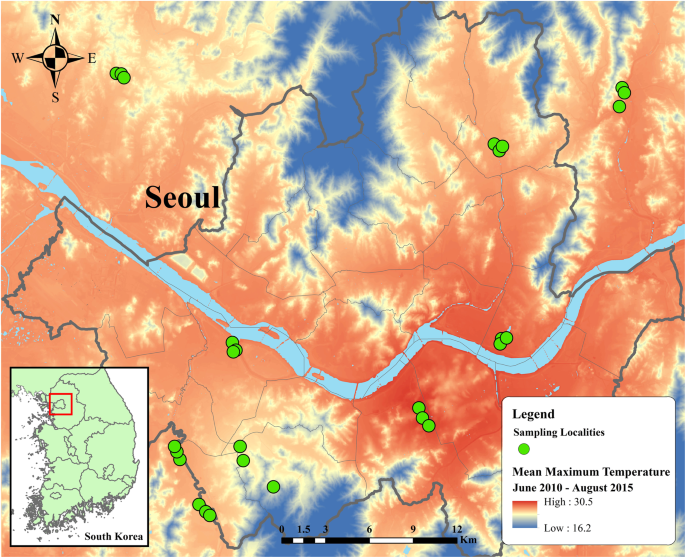
Nguyen, H. Q., Andersen, D. K., Kim, Y. & Jang, Y. Urban heat island effect on cicada densities in metropolitan Seoul. PeerJ 6, e4238 (2018).
Lobser, S. & Cohen, W. MODIS tasselled cap: land cover characteristics expressed through transformed MODIS data. International Journal of Remote Sensing 28, 5079–5101 (2007).
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology: A Journal of the Royal Meteorological Society 25, 1965–1978 (2005).
Tzavali, A., Paravantis, J. P., Mihalakakou, G., Fotiadi, A. & Stigka, E. Urban heat island intensity: a literature review. Fresenius Environmental Bulletin 24, 4535–4554 (2015).
Sanborn, A. F. & Phillips, P. K. Thermal responses of the Diceroprocta cinctifera species group (Homoptera: Cicadidae). The Southwestern Naturalist, 136–139 (1996).
Sanborn, A. F. & Heath, J. Thermal responses of periodical cicadas: within and between brood parity (Hemiptera: Cicadidae: Magicicada spp.). Open Access Insect Physiol 1, 13–20 (2009).
Sanborn, A. F., Heath, J. E., Heath, M. S. & Phillips, P. K. Thermal adaptation in North American cicadas (Hemiptera: Cicadidae). Journal of thermal biology 69, v–xviii (2017).
Sanborn, A. F., Phillips, P. K., Heath, J. E. & Heath, M. S. Influence of altitude, habitat and microhabitat on thermal adaptation of cicadas from Southwest Texas (Hemiptera: Cicadidae). Journal of Thermal Biology 36, 386–389 (2011).
Sanborn, A. F., Heath, J. E. & Heath, M. S. Thermoregulation and evaporative cooling in the cicada Okanagodes gracilis (Homoptera: Cicadidae). Comparative biochemistry and physiology. Comparative physiology 102, 751–757 (1992).
Harrison, J. F., Woods, H. A. & Roberts, S. P. Ecological and environmental physiology of insects. Vol. 3 (Oxford University Press, 2012).
Toolson, E. C. Water profligacy as an adaptation to hot deserts: water loss rates and evaporative cooling in the Sonoran Desert cicada, Diceroprocta apache (Homoptera: Cicadidae). Physiological Zoology 60, 379–385 (1987).
Rao, C. R. The use and interpretation of principal component analysis in applied research. Sankhyā: The Indian Journal of Statistics, Series A, 329–358 (1964).
Oksanen, J. Multivariate analysis of ecological communities in R: vegan tutorial. R Doc 43, 11–12 (2015).
Sanborn, A. F., Phillips, P. K., Heath, J. E. & Heath, M. S. Comparative thermal adaptation in cicadas (Hemiptera: Cicadidae) inhabiting Mediterranean ecosystems. Journal of Thermal Biology 36, 150–155 (2011).
Lee, Y. A list of Cicadidae (Homoptera) in Korea. Cicada 15, 1–16 (1999).
Sanborn, A. F., Heath, J. E., Phillips, P. K., Heath, M. S. & Noriega, F. G. Thermal adaptation and diversity in tropical ecosystems: evidence from cicadas (Hemiptera, Cicadidae). PLoS One 6, e29368 (2011).
Sanborn, A. Comparative thermoregulation of sympatric endothermic and ectothermic cicadas (Homoptera: Cicadidae: Tibicen winnemanna and Tibicen chloromerus). Journal of Comparative Physiology A 186, 551–556 (2000).
Van Voorhies, W. A. On the adaptive nature of Bergmann size clines: a reply to Mousseau, Partridge and Coyne. Evolution 51, 635–640 (1997).
Crill, W. D., Huey, R. B. & Gilchrist, G. W. Within-and between‐generation effects of temperature on the morphology and physiology of Drosophila melanogaster. Evolution 50, 1205–1218 (1996).
Ray, C. The application of Bergmann’s and Allen’s rules to the poikilotherms. Journal of morphology 106, 85–108 (1960).
Dale, A. G. & Frank, S. D. Warming and drought combine to increase pest insect fitness on urban trees. PloS one 12, e0173844 (2017).
Musolin, D. L., Tougou, D. & Fujisaki, K. Too hot to handle? Phenological and life-history responses to simulated climate change of the southern green stink bug Nezara viridula (Heteroptera: Pentatomidae). Global Change Biology 16, 73–87 (2010).
Kingsolver, J. G. & Huey, R. B. Size, temperature, and fitness: three rules. Evolutionary Ecology Research 10, 251–268 (2008).
Brans, K. I. et al. The heat is on: Genetic adaptation to urbanization mediated by thermal tolerance and body size. Global change biology 23, 5218–5227 (2017).
Geerts, A. et al. Rapid evolution of thermal tolerance in the water flea Daphnia. Nature Climate Change 5, 665 (2015).
Oberg, E., Del Toro, I. & Pelini, S. Characterization of the thermal tolerances of forest ants of New England. Insectes sociaux 59, 167–174 (2012).
Baudier, K. M., Mudd, A. E., Erickson, S. C. & O’donnell, S. Microhabitat and body size effects on heat tolerance: implications for responses to climate change (army ants: Formicidae, Ecitoninae). Journal of Animal Ecology 84, 1322–1330 (2015).
Bennet-Clark, H. & Young, D. The scaling of song frequency in cicadas. Journal of Experimental Biology 191, 291–294 (1994).
Sueur, J. & Aubin, T. Specificity of cicada calling songs in the genus Tibicina (Hemiptera: Cicadidae). Systematic Entomology 28, 481–492 (2003).
Shieh, B.-S., Liang, S.-H., Liao, C.-Y. & Chiu, Y.-W. Song frequency correlates with latitude and individual body size in the cicada Mogannia formosana Matsumura (Hemiptera: Cicadidae). acta ethologica 20, 147–155 (2017).
Sanborn, A. F. Body temperature and the acoustic behavior of the cicada Tibicen winnemanna (Homoptera: Cicadidae). Journal of insect behavior 10, 257–264 (1997).
Source: Ecology - nature.com



