Asian citrus psyllids
An uninfected (CLas-free) colony of D. citri was maintained at the U.S. Horticultural Research Laboratory, Fort Pierce, FL. The colony was routinely tested to confirm that it was HLB-negative using previously reported PCR methods50. Psyllids were reared on seedlings of a susceptible host, Citrus macrophylla Wester, and maintained at 28 °C, 14:10 L:D. All insects used for bioassays were adults between 8 and 10 days old; sex ratio was approximately 50:50. All cage experiments were performed in a temperature (26 °C) and humidity (60–65% RH) controlled walk-in chamber under 14:10 L: D conditions. The D. citri used for the yellow sticky trap assays were reared in a different greenhouse under similar conditions and protocols at the US Department of Agriculture, Agricultural Research Service, Center for Medical, Agricultural, and Veterinary Entomology in Gainesville, Florida. The host plant used for rearing D. citri in Gainesville was orange jasmine, Murraya paniculata (Linnaeus). The greenhouse was climate controlled at 29 ± 3 °C under a photoperiod of 16:8 L:D using natural light and metal halide lamps. Plants were watered 3 times weekly and fertilized once a month with one tsp of Milorganite® plus (20-20-20 fertilizer solution). As in Fort Pierce, FL, both rearing plants and randomly selected psyllids from the colony were verified to be free of CLas infection using previously described PCR methods50. Adult D. citri used for assays from the Gainesville colony represented a mix of ages and physiological states.
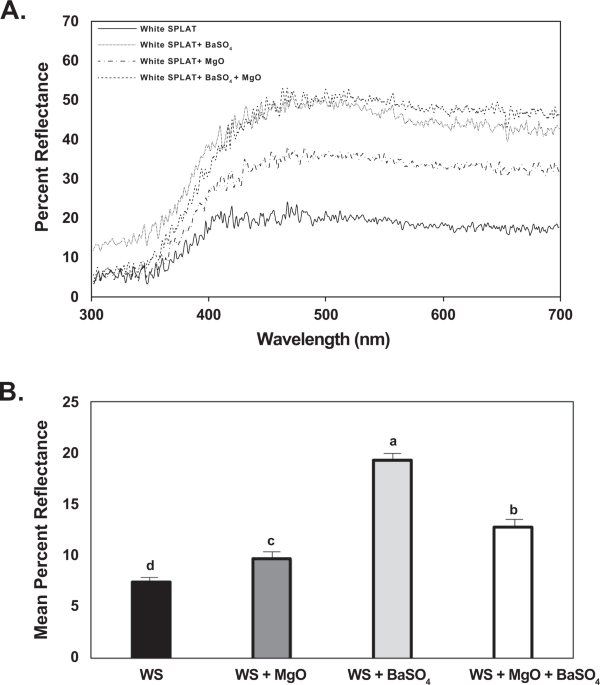
Measurement of UV reflectance from magnesium oxide and barium sulfate
The irradiance spectra of various artificial light sources used in SPLAT probing assays and solar radiation was measured using a concave grating spectrometer (UV-VIS BLACK-Comet, StellarNet Inc, Tampa, FL) over the spectral range of 350–700 nm. The light sources were a standard fluorescent bulb (F40T12/CW Plus, 40 Watt, Philips, USA), metal halide-sodium bulbs (GE Saf-T-Gard Multivapor Quartz Metal Halide ED37, MVT400/I/U; and GE Lucalox Sodium LU400/DX), and unfiltered, natural sunlight. A second concave grating spectrometer was used for the reflectance spectra that had a spectral range of 300–700 nm (UV-VIS BLACK-Comet, StellarNet Inc, Tampa, FL). The reflectance spectra of sticky traps/visual targets used in indoor bioassays and field trials was obtained with the aid of a light source that had a spectral range of ultraviolet and visible light (Deuterium-Tungsten Halogen Light Source, StellarNet Inc, Tampa, FL). A reflectance probe connected to the light source and possessing six illuminating fibers and one read fiber (RPH1 Reflectance Probe, StellarNet Inc, Tampa, FL) was mounted on a holder (RPH1, StellarNet Inc, Tampa, FL) and maintained at a 45° angle at a set distance of 1.9 cm from the sticky traps/visual targets. All measurements were standardized using a white reflectance standard (RS-50, StellarNet Inc Tampa, FL) and a dark reflectance standard obtained by turning off the shutter in the light source. For each measurement of visual targets, two measurements were obtained and then averaged. Only one measurement was performed to quantify the reflectance spectra of the powdered form of magnesium oxide and barium sulfate.
Visual attraction to yellow sticky cards with different rates of magnesium oxide
All traps consisted of colored sticky cards (10 × 10 cm) prepared from foamboard and painted with selected colors on one side. A hanger for traps consisted of a large paper clip with one end straightened to embed into the foamboard in the center top. The other end was hooked into screens at the back-top seam of the cage using safety pins. Traps were painted with a base white coat (Zinzer 123 primer, Rust-o-leum, Vernon Hills, IL) and then coated with a UV-reflecting yellow bird decoy paint (ReelWings Decoy Co., Inc., Fargo, ND). Sticky adhesive (Tangle-trap®, Tanglefoot, Grand Rapids, MI) was thinly coated on the traps to collect psyllids. Four rates (0 mg, 0.25 mg, 0.5 mg and 1.25 mg) of MgO were compared by mixing with the adhesive before applying on to the sticky cards. The powder was weighed and evenly admixed into the sticky adhesive before application on the traps.
Assays were conducted in the laboratory at 29 ± 2 °C and 40–50% RH under illumination from metal halide lamps (150 W) in digital ballasts. Multi-choice assays were conducted in large screened cages (MegaView Science, Taichung, Taiwan) that were 45 cm tall × 90 cm long × 45 cm wide. Cages had screened sides and clear plastic tops. Traps to be tested were placed equidistantly between cage sides and other traps. The surface of one side of the traps was covered with clear sticky film (Alpha Scents, West Linn, OR). About 100–120 psyllids were released into the cage after traps were placed in the cages. Assays lasted 5 h with psyllids released into cages 0800 hr. After assay completion, traps were removed from the cage and remaining psyllids were aspirated, counted, and sexed. Traps were examined under 10-40x magnification to sex and count psyllids. Data are presented as percentage of total responding psyllids that were caught on each trap. Assays were replicated 12 times. Data were analyzed using analysis of variance (ANOVA) followed by Tukey’s HSD for mean comparisons.
Effect of magnesium oxide and barium sulfate on probing by D. citri under different UV conditions
A completely randomized choice assay was used to study the probing of D. citri in response to chemosensory stimuli (odorants and/or tastants) as described previously6,38,51. This assay measured insect orientation within a screened cage to combinations of color and texture, and subsequent probing behavior that may result from a combination of olfaction and gustation upon contact with a wax substrate containing magnesium oxide or barium sulfate. Test compounds were incorporated into a slow-release wax matrix (SPLAT™, ISCA Technologies Inc., Riverside, CA) and offered to caged D. citri adults in beads of yellow or white SPLAT. A yellow wax substrate was prepared by adding 6 µl of green food coloring (McCormick & Co., Inc., Hunt Valley, MD, USA) to 10 gm of white SPLAT provided by the manufacturer resulting in a yellow-green mixture. Yellow or white stocks were then combined separately with magnesium oxide/barium sulfate powders in a vortex rotor for 5 min. Mixtures were 1% by weight with 100 mg of magnesium oxide or barium sulfate added to 10 gm of SPLAT. One ml of each treatment (white or yellow SPLAT with or without magnesium oxide/barium sulfate) was applied as narrow strips of beads (2.0 × 0.5 × 0.1 cm) to 6 glass cover slips (22 × 22 mm, Fisherbrand Microscope Cover Glass 12-542-B). Each cover slip received approximately 0.17 ml of wax, which was air dried for 18 h. A total of eight treatments were tested as a choice experiment in the initial trials. SPLAT bead treatments compared were: white, blank control; white, magnesium oxide; white, barium sulfate; white, magnesium oxide + barium sulfate; yellow, blank control; yellow, magnesium oxide; yellow, barium sulfate; yellow, magnesium oxide + barium sulfate. The beads were placed in a completely randomized pattern with 5 replications on the floor of a cubical cage (60 × 60 × 60 cm, BioQuip, San Diego, CA). Cages were replicated 4 times and treated as blocks. The same design was used in each cage but with unique randomizations of treatments within each cage.
Cohorts of 250 8- to 10-d-old D. citri adults were starved for 3 h and then released into each cage and allowed to move freely and probe into the wax beads within the cage for 22 h. Cages were held in a temperature and humidity controlled incubator at 26 °C, 75% RH and continuous light. Experiments were performed under various low and high UV (UV chamber), as well as, unfiltered, natural sunlight conditions to quantify the effect of UV reflectance from the surface of SPLAT beads on psyllid attraction and probing behavior. Experiments under low UV conditions were performed under fluorescent lights (F40T12/CW Plus, 40 Watt, Philips, USA). SPLAT probing experiments under complete UV conditions were performed in a UV chamber containing sodium vapor lamps and metal halide lamps (GE Saf-T-Gard Multivapor Quartz Metal Halide ED37, MVT400/I/U; and GE Lucalox Sodium LU400/DX). The same experiments were performed outdoors under unfiltered, natural sunlight conditions during tdaylight hours (11:00-17:00). SPLAT beads from the 8 treatments were placed in a completely randomized pattern with 5 replications on the floor of a cubical cage (60 × 60 × 60 cm, BioQuip, San Diego, CA). Cages were replicated 4 times and treated as blocks. Cohorts of 250 8- to 10-d-old D. citri adults were starved for 3 h and then released into each cage and allowed to move freely and probe into the wax beads for 6 h. The time was limited to 6 hours of highest UV daylight.
To visualize salivary sheaths produced by feeding attempts on the wax beads, cover slips were removed from the cages and beads were stained with Coomassie blue dye for 60 sec for yellow SPLAT beads and 45 sec for white beads, washed in water and allowed to air-dry6,38,51. The number of salivary sheaths in each bead was counted under a stereomicroscope at 50 X magnification. The difference in number of salivary sheaths between treatments were analyzed by ANOVA followed by Tukey’s HSD for comparison of means.
Effect of phagostimulant blend with magnesium oxide on probing choice
Phagostimulant compounds can induce feeding and such compounds could act as nutrients or token stimuli for insects. Our earlier studies have reported that a phagostimulant blend of formic acid: acetic acid: p-cymene in the ratio 3.5:1.6:1 can increase probing behavior by D. citri as measured by greater deposition of salivary sheaths38. In this experiment, we tested how addition of magnesium oxide to SPLAT containing phagostimulant blend affects probing behavior and salivary sheath secretion by psyllids. A choice assay was performed using yellow SPLAT beads containing magnesium oxide with and without phagostimulant blend. Experiments were performed in a temperature and humidity controlled UV chamber at 26 °C, 75% RH and continuous light. Treatments compared were: yellow SPLAT beads, yellow beads containing magnesium oxide, yellow beads containing the phagostimulant blend, and yellow beads containing magnesium oxide + the phagostimulant blend. One ml of each treatment (yellow SPLAT with or without MgO/phagostimulant blend) was applied as a narrow strip of beads (2.0 × 0.5 × 0.1 cm) to 6 glass cover slips (22 × 22 mm, Fisherbrand Microscope Cover Glass 12–542-B). Each cover slip received approximately 0.17 ml of wax and 0.67 μl of the phagostimulant blend, or 1.6 mg of magnesium oxide or a combination of both depending on the treatments. The beads were air-dried for 2 h prior to assays. Treatments were arranged in a completely randomized pattern on the floor of a cubical cage (60 × 60 × 60 cm, BioQuip, San Diego, CA). Cohorts of 250 8- to 10-d-old D. citri adults were starved for 3 h and then released into each cage and allowed to move freely and probe into the wax beads within the cage for 22 h. Cages were replicated 4 times and treated as blocks. Means were compared by Tukey’s HSD following a significant ANOVA.
Source: Ecology - nature.com



