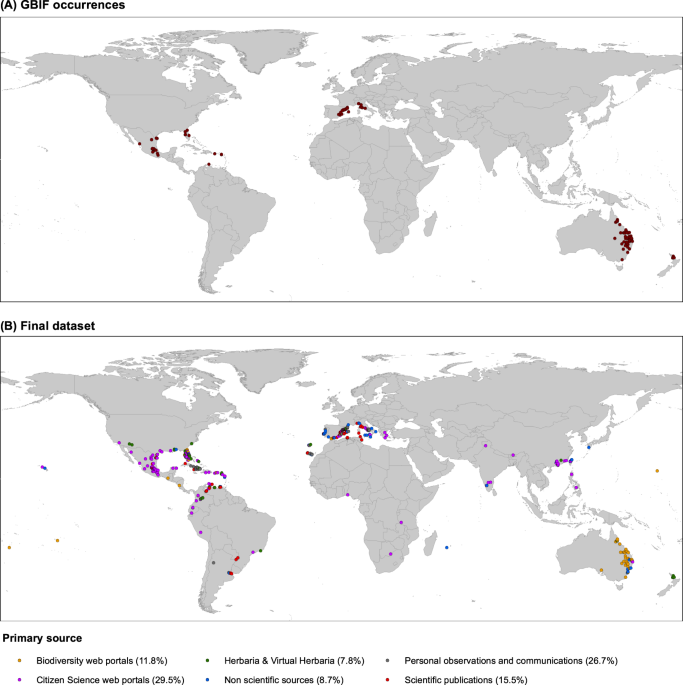
Global occurrences: standard sources vs. citizen science
Using exclusively “standard” sources including biodiversity web portals, herbaria, scientific publications, personal observations, and personal communications to search for documented locations would lead to large distributional biases. Indeed, using exclusively standard sources would imply that more than one third of them would be lost. Our results emphasize the role that citizen science plays in collecting biodiversity data, as generally agreed63,64. Citizen science web portals such as iNaturalist provided a large quantity of location data for K. × houghonii (Fig. 1B; Supplementary Table S1). Subsequently, such databases might constitute a primary source for biodiversity knowledge when high-quality photographs allowing for identification of the species and habitat or other voucher-essential data (collector, date, collection locality, and geographic coordinates) are provided to ensure the plant is wild and not cultivated.
Colonization history: temporal and geographic expansion
It is significant that K. × houghtonii has reached a nearly worldwide distribution in just 80 years, being present on all the continents except Antarctica. Its creator, A. D. Houghton, already noted its potential as an experimental plant for genetic, commercial, and horticultural purposes4. Indeed, it seems that the plant was already common as a horticultural plant in the United States during late 1940s together with other species of the genus14. Experimental studies in K. × houghtonii outside of the United States were reported soon after, as those of F. Resende and colleagues carried out in Portugal in the 1950s aimed to study the consequences of hybridization11,12. From the 1980s, studies relating to their toxicity and medicinal potential started to be published (e.g.65,66). The first escapees of this plant into the wild are likely to have originated from research centers or horticultural greenhouses. In recent years, however, domestic gardens are the most apparent source of incursions into the wild, particularly due to its popularity as a garden plant, at least in North America7, Australia67, and Europe8,16. For example, in Spain and Italy, K. × houghtonii has become a serious and invasive problem, likely stemming from its popularity as an ornamental plant owing to its low maintenance (C. Gómez-Bellver and A. Stinca, pers. obs.). More recently it has become popular in China where it is commonly sold in markets (J. López-Pujol et al., pers. obs.).
Notwithstanding K. × houghtonii’s probable first escape from cultivation in the United States, the first recorded escapee(s) of this hybrid were from 1965 in Byrnestown (Queensland, Australia), albeit from an herbarium sheet without leaves (G. Brown, Queensland Herbarium, pers. comm.), so with uncertain identification as K. × houghtonii. The first record of the hybrid that we can confidently state as occurring in the wild is also from Australia, in this case from New South Wales collected in 1970 (Appendix). Although slightly earlier records may exist, as there are a few specimens dating from 1966–1969 also from Queensland, these were on loan at the time when we requested for scanned specimens. In Australia, K. × houghtonii soon became a serious invader and, by the late 2000s, it was already widespread throughout the eastern part of the country, reaching as far as southern regions (Fig. 2). At present it is listed as an invasive species in Australia67,68,69, where it often produces large infestations that can be accompanied by cattle poisoning episodes65. It has been present in New Zealand since at least the mid-1980s, but exclusively affecting the northern tip of the archipelago (Fig. 2B). The presence of the species is also confirmed in other parts of Oceania, including Micronesia (Wake Island) and Polynesia (Tonga, French Polynesia, and Hawaii; Fig. 2).
In addition to Oceania, America is the only region with occurrences confidently assigned to K. × houghtonii before the 1990s (Fig. 2B). The first recorded wild locality is from the Bahamas in 1975, as shown in a specimen preserved in the Steere Herbarium (NY 1515254; Appendix). Surprisingly, the first confident observation in the United States is as late as 1988 in Florida, although according to Mild70 the nothospecies would have been present in Harlingen (Texas) since the 1960s. Until the 2000s all occurrences were restricted to Florida, with its hypothetical expansion out of (but also within) this state having occurred in recent years. These increases appear to have coincided with its expansion in other parts of the world (Appendix; see also below). In Florida, this spread has merited its recent inclusion in the Florida Exotic Pest Plant Council Invasive Plant List71. In Venezuela, there are several records for the period 1979–1984 (Appendix). The presence of such ornamental plants escaping from cultivation in this country should not be regarded as exceptional, given the correlation between consumption of ornamental plants and gross domestic product (GDP) per capita72. Until the early 1980s, Venezuela was regarded as one of only four Latin American countries with an upper-middle-income economy73, with a GDP per capita higher than those of Portugal or South Korea (https://data.worldbank.org/). Although in some places of Central and South America (Anguilla, Colombia, Ecuador, Guatemala, Nicaragua, and Puerto Rico) the nothospecies was recorded before the year 2000, K. × houghtonii apparently had an “outbreak” in recent years (Appendix). Such an apparent explosion could have been due more to sociological reasons such as the rise in citizen science data collection and an increase in the use of ornamental plants in recent years throughout South America, rather than ecological or climatic ones. In Mexico, second only to the USA in the number of confirmed occurrences, all recorded localities of K. × houghtonii are much more recent (2012–2018), and are certainly linked to the launch of iNaturalist, with 66 out of 70 occurrences taken from this source (Appendix).
Kalanchoe × houghtonii did not apparently reach the Old World until more recently, with the first confirmed record in Europe being in 1996 from eastern Spain (Fig. 2C). Since then, the number of records in Europe and particularly on the Iberian Peninsula have increased exponentially (Appendix), partly because of the extensive network of local botanists but also due to its popularity in horticulture. However, this hybrid is not listed in the Spanish catalogue of invasive alien species. In Africa, the nothospecies would likely have arrived shortly after those to Spain, with the first observations being from Madeira Island in 1999, whilst the first occurrences for continental Africa coming almost a decade later, in Tunisia (Appendix). It should be noted the greater abundance in observations coming from the Portuguese (Madeira Archipelago) and Spanish (Canary Islands) outermost regions, in contrast to the scarcity of records for continental Africa. Such a disparity is likely due to the lower level of botanical knowledge on the African continent and particularly throughout sub-Saharan Africa74,75, combined with the low consumption of ornamental plants.
According to our data, Asia would be the last continent where K. × houghtonii spread to. All gathered Asian localities have been recorded since 2006 with a single exception; a population in Taipei, Taiwan Island (Fig. 2C; Appendix). The fact that many of the Chinese localities are from Hong Kong and Taiwan is not surprising, as these areas can be regarded as gateways for the introduction of alien species in China; being the primary places of first detection for many non-native species76,77.
Although a study aimed to discern whether the ecological niche of K. × houghtonii is broader than those of its parental species is underway, our field observations in Spain, where the three species are present, seems to suggest it; populations of K. × houghtonii are usually larger and more common than those of the parentals. Certainly, the nothospecies can produce large infestations, that in some cases may derive into dense monospecific phytocoenosis. Under favorable conditions (open, sunny areas under relatively dry or even semiarid conditions, on sandy or rocky soils; see below), densities may reach 1000–2000 individuals/m2 when plantlets and seedlings are produced19,22 (Supplementary Fig. S3E). These conditions probably mirror those of the parental species in Madagascar, where they grow on granite, sandstone or limestone outcrops, or coastal and inland unconsolidated sands5,78,79. In Cerro Saroche, Venezuela, a semiarid site with a typical xeric environment composed of spiny scrubs and thorny forests, the nothospecies has spread over an area of ca. 20 ha thanks to a very vigorous population growth (rate of growth, λ = 4.06), potentially allowing the population to quadruple in size every year. Such estimates are based on recruitment of asexual plantlets, due to the asexual plantlets ability to reproduce in less than one year. In contrast, sexual seedlings require a minimum of three years to reproduce19. According to Herrera et al.19, this strategy allows quick population growth during the initial phases of invasion, when populations are more susceptible to Allee effects or to post-introduction demographic bottlenecks. This strategy increases the probability of establishment and reduces the opportunities for effective control of K. × houghtonii. Similar results have been obtained for a population from Barranca de Metztitlán in Mexico, although for this population no sexually reproduced specimens were observed, and growth rates are lower than in Venezuela but still high (λ = 1.36)22. Thus, such a demographic strategy may have allowed the considerable global establishment of K. × houghtonii, as also occurs in other plants with a vegetative propagation strategy80,81.
Potential distribution at present: climatic conditions and geographic regions where the species could inhabit
The most suitable conditions for K. × houghtonii are warm and dry climates. Low cold-tolerance is one of the most limiting factors of Crassulaceae and in general all succulent species with a CAM (Crassulacean Acid Metabolism) photosynthetic strategy. The lowest tolerance temperatures detected for this group of plants are −10 °C for Opuntia ficus-indica (L.) Mill. and −24 °C for O. streptacantha Lem.82. These two species, however, present special adaptations related to sugar accumulations that are necessary to prevent the intercellular ice crystal formation82,83,84, a set of specialized physiological traits that has not been observed in K. × houghtonii. The lack of sub-freezing acclimation during the coldest year periods (as bio6 showed; Fig. 3) could indeed be the most hindering factor for K. × houghtonii to reach higher latitudes, and greater altitudes.
Kalanchoe × houghtonii has a low water demand for its survival (Fig. 3). This is in accord with its ecophysiological traits, since CAM plants are highly tolerant and adapted to periodic droughts, salinity, or even elevated temperatures85,86. In fact, the stomatal opening during night-time instead of daytime offers a lower transpirational water loss84, which is a clear evolutionary adaptive advantage of increased water use efficiency and maintenance of considerable internal water reserves. However, it should be noted that the nothospecies would not be able to survive in geographic regions showing extremely irregular seasonal rains with extended periods of drought (e.g. semi-desert or desert). CAM plants need regular precipitation throughout the year or, at least, rains that are periodical and predictable; if they lose more than 50% of their total water reserves, they would perish84,87,88,89. Locations with too-high humidity (e.g. in tropical wet climates) would not be suitable for K. × hougthonii either, due to the stem and leaf cells being unable to release water fast enough into the atmosphere under such conditions. Once the maximum capacity of water storage is reached, plant cells may burst due to excesses of turgor pressure. Such an effect is well-described in succulent gardening when plants are over-watered, and their leaves may have a water-soaked, translucent appearance90. Nevertheless, precipitation of the coldest quarter (bio19) is the most important factor regarding the rainfall regime delimiting the potential distribution of K. × hougthonii. As this nothospecies does not have the sugar-mediated osmoregulation mechanism to control freeze dehydration of some low-temperature acclimated cacti82, a high-precipitation regime would produce the lowering of intracellular osmotic pressure, thus promoting the diffusion of intracellular water into the apoplastic spaces where ice crystals are formed82. The effects of cellular freeze dehydration is also a well-known phenomenon in succulents gardening—“weather that is most threatening to succulents is rain followed by frost”91. This is also the reason why watering should be restricted during winter in Kalanchoe spp. cultivation6.
Antropogenic pressures impacting potential distribution
To our knowledge, K. × hougthonii shows the highest weight for HF in niche models (Supplementary Table S3) for any invasive plant; permutation importance in invasive plants is generally below 10%92. Such a high percentage is, however, not surprising given the species’ low dispersal capabilities; the overwhelming predominance of clonal growth through bulbils making the spread of K.× hougthonii a process strongly linked to humans. Undoubtedly, anthropogenic pressures influence the distribution of alien species in their non-native ranges along the several invasion stages (transport, introduction, establishment, and spread93), usually enhancing their expansion directly (e.g. deliberate or accidental releases94) or indirectly (e.g. via urbanization and land-use change95).
Initially, we expected the addition of the HF variable to ENMs would increase the suitable areas for K. × houghtonii, as previously detected for other cases such as Acacia farnesiana (L.) Willd96. In contrast, this expectation was not fulfilled in our case (the model performed without HF predicted around 25% more suitable ranges; Table 1 and Fig. 5). The presence of K. × houghtonii may be limited to the origin foci (mostly in private, and less-frequently, in public gardens) and adjoining areas, while climatically suitable regions far from human influence would not likely to be invaded. It should be noted that K. × houghtonii seems to be unable to disperse long distances, since sexual reproduction is quite unsuccessful: only a small proportion of produced seeds are viable (17.9%), with very low germination success (11.9%), and the survival rates of sexually-produced seedlings is also extremely low (10%20). In addition, the propagules mostly germinate in situ, just beneath or very near the mother plant. Indirect estimates of dispersal rates in Cerro Saroche (Venezuela) suggest that dispersal distances are generally limited20, although episodic floods that are produced during the rainy season can disperse the plantlets to relatively longer distances (I. Herrera, pers. obs.). Overall, the addition of HF variable to ENMs allowed us to obtain much more refined models for the potential distribution of the nothospecies (Fig. 5), which highlights the need to include this input element when distribution modelling for invasive species. The representation of K. × houghtonii occurrences on the map of “red alert” areas (Fig. 6A) suggests that the nothospecies could largely expand its current range.
How do different levels of occurrence data accuracy influence ENM? Is it really necessary a multi-source search of occurrence data and an accurate filtering step to perform ENM?
In general, results shown in Table 2 suggest that a direct use of GBIF records could be appropriate without an extensive exploration of other sources and a taxonomic expertise validation process in covering the realized environmental species niche requirements. Nevertheless, it could be highly dependent on the target taxonomic group, as for primates, the comparison of datasets without or with expert knowledge resulted in the recovering of a higher number of outliers in non-expert group97.
Unexpectedly the ENMs performed with Raw GBIF and Final occurrence datasets resembled greatly (Table 2 and Fig. 7). In short, the resulting ENMs does not seem to be largely influenced by the occurrence data used, which might be due to the fact that species like K. × houghtonii and most Crassulaceae have a “very defined” interval range of climatic conditions; i.e. the tolerance to temperature and precipitation out of the optimal range is very low (see Fig. 3), and the shape of the response curves of bio17 and bio19 variables is more or less flat-topped and decline abruptly towards the margins (Supplementary Figs. S6 and S7). Conversely, in a study that tested the effect of spatial bias in GBIF records of Eurasian butterfly, the authors reported a decline of model quality with increased spatial bias44.
Careful observation of Fig. 7 shows, however, that there are areas not predicted using the Raw GBIF dataset (green ranges in Fig. 7), especially from equatorial climatic zones. This may be due to the fact that K. × houghtonii records located in these areas and in general for equatorial climatic zones are mostly gathered from citizen science websites (like iNaturalist; Fig. 1B) and not yet incorporated into GBIF. The lack of records from equatorial climatic zones on GBIF may simply respond to the nature of GBIF itself, as it is a research infrastructure funded by the world’s governments. Therefore, biodiversity data uploaded greatly depends on the node of every country. Many countries located in equatorial zones are non-participating countries in GBIF. This may be attributed to being low-income economies, although other reasons may apply (e.g. political). However, as noted by Amano and Sutherland98, even in participant countries, the amount of data provided may be low because the number of GBIF records per square kilometer depends greatly on GDP per capita (i.e. economic wealth), the number of English speakers, and country’s level of security. All these parameters rank generally very low in equatorial countries, which have hampered access to adequate funding for research centers and the work of researchers itself. Additionally, even in the case that citizen science portal websites are working well in a given country by providing lots of occurrences (e.g. PPBC in China), these data may not reach GBIF if there are no nodes submitting or endure long delays if nodes are not working properly.
Our results also show an overprediction of the nothospecies’ range with the Raw GBIF dataset (red areas in Fig. 7). Other studies comparing unfiltered vs. filtered presence datasets reported similar results. For example, false levels of species richness were recovered on large ecoregions for the plant tribe Cinchoneae (Rubiaceae)99 or wider elevational extents for three species of Phaedranassa (Amaryllidaceae)100.
A multi-source search and expert verification of presence records, aimed to avoid taxonomic (i.e. misidentifications) and spatial (i.e. georeferencing errors) biases, is generically regarded as critical to research focused on identification of current species location101 and evaluation of the conservation status of a given species (e.g.102). On the contrary, considering the results found here, one may think that these previous processes would not be necessary in ENM studies with a biogeographical global focus, as the “big picture” using Raw GBIF and final datasets is similar. Graham et al.103 also concluded that ENMs are particularly robust to moderate levels of errors in occurrence databases. Nevertheless, using exclusively GBIF data as a way to reduce time and money is only relatively justified for species distributed across well explored large-scale ranges and regions containing GBIF data providers. It should be noted that ENM approaches conducted for those species distributed in areas that do not provide data to GBIF, or that do not do it promptly, could be more susceptible to produce biased results. In addition, a detailed inspection of Fig. 7 shows that large regions of countries and even entire countries were not predicted as suitable using the Raw GBIF dataset, which eventually would have serious effects on the future management of K. × houghtonii in case of invasion; for example, regions or countries not suitable for the species would not implement policies of early detection/early warning. This suggests that making a complete multi-source location search, particularly including citizen science and other online databases, followed by a refining step is of critical importance in ENMs with management or conservation ends.
Effects of climate change on global potential future distribution
A considerable reduction of K. × houghtonii’s current range (Table 1) could occur due to the lack of adequate climates, including many locations (Supplementary Table S3). The geographic regions predicted to be lost with respect to present scenarios is presented in Fig. 8 (red coloration). As a general pattern, it was observed that: (1) the lower the latitude, the more areas lost; and (2) most of the lost areas are continental, whereas many coastal areas are maintained. The latter observation agrees with multi-species studies of plant invaders in the United States (896 spp.)104 and Australia (72 spp.)105, and probably reflects that continental areas would tend to undergo more severe climatic changes than coastlines.
On the other hand, new suitable areas are predicted under future climate scenarios, which are mainly located in higher latitudes on both northern and southern hemispheres (Fig. 8). One clear example where the species cannot survive under present conditions is northern Europe, which would become climatically suitable. Such predicted latitudinal migration, with a poleward shift for K. × houghtonii, is interestingly following one of the most typical and well documented effects of climate change on species distribution106,107. High similarities were detected between results found here and other global assessments of future distributions of the top 100 worst invasive species performed by Bellard et al.108. According to these authors, tropical regions at low latitudes would experience the greatest decrease in potential number of invasive species, while the expanded ranges were in temperate regions such as northern Europe. In agreement with this, a recent study of 783 ornamental alien species planted in European gardens but not yet naturalized, observed that under a warming climate the hotspots of naturalization and invasion risk would considerably increase in northern and eastern parts of the European range109.
Considering the marked invasive behavior and the relatively wide temperature and precipitation ranges shown by the species (Fig. 3), one might expect future increase in its distribution range. On the contrary to this expectation, future projections suggest that the plant would be negatively affected by the ongoing climate change. Other global plant invaders have also shown potential future range contractions, for example a 32% reduction was observed for the grassland weed Nassella neesiana (Trin. & Rupr.) Barkworth due to increases in temperature leading to lethal heat stress110. As far as we know, the present study is the first estimate of global range shifts under a scenario of climate change not only for a Crassulaceae species but also for CAM species. Studies carried out at local or regional levels have yielded equivocal results: some show a range expansion such as Kalanchoe × houghtonii in NW Spain16 and Echinocereus reichenbachii (Terscheck ex Walp.) J. N. Haage in the United States111, whilst for others a range reduction was detected, e.g. Kalanchoe tubiflora and Pereskia aculeata Mill. in eastern Australia112 or Coryphantha werdermannii Boed. in northern Mexico113. Looking towards future research projects, it would be interesting to test whether a decrease in potential habitats might constitute a general pattern for Crassulaceae and CAM plants.
Shrinkage of potential areas for the year 2070 compared to the present in K. × houghtonii might be related to its ecophysiological traits. The extraction of climatic values of lost and gained areas in respect to future/present ranges revealed that K. × houghtonii would tend to be displaced to regions with low precipitation values. Inversely, regions with high rainfall values would be abandoned (see bio16, bio17, and bio19 in Supplementary Fig. S11). In contrast, the temperature ranges where it inhabits at present, both for minimum and maximum limits, would be maintained without significant modifications in the year 2070 (see bio5, bio6, and bio8 in Supplementary Fig. S11). As noted previously it seems that K. × houghtonii would not be able to withstand extended long wet periods, particularly during cold annual intervals. The combination of water storage saturation and freezing temperatures may injure succulent plants82. Thus, at least for K. × houghtonii, climatically suitable areas for the year 2070 are significantly reduced, because part of the current potential ranges would become unsuitable due to an excess of rainfall. This is in accordance with global trend changes, of which a significant rise in extreme periods of prolonged wet cycles have been estimated114,115.
Ultimately, we explored where the most likely potential invasion hotspots over present and future time slices could be located. This was achieved through the intersection of the eight ENMs performed which consisted of present scenario with and without HF plus six future scenarios (Fig. 6B). With this approach, firstly we observed that the main current presence locations of K. × houghtonii would still appear as suitable (e.g. Florida, Río de la Plata region, the Mediterranean Basin, the Chinese province of Guangdong, Taiwan Island, and western Australia). Secondly, we observed several areas where the species has not been documented to date but potentially have a high success rate of invasion. These areas could be regarded as “red alert areas”, and include California, central Chile coastline, most of the Atlantic coast of Brazil, Uruguay, northern Argentina, north-western Iberian Peninsula, the Atlantic coast of France, Corsica, Azores Islands, Crete, East African mountains, parts of Madagascar, South Africa, southern continental China, southern Australia, Tasmania, and New Caledonia (Fig. 6B).
Conclusions and future prospects
In this study, we analyzed for the first time the global geographic distribution of the invasive plant Kalanchoe × houghtonii. The main conclusions drawn from this study are as follows: (1) a multi-source data compilation process was key in allowing us to infer the fast and complex expansion patterns shown by this hybrid; (2) the results of ENM (to our knowledge, the first one carried out with a CAM taxon on a worldwide scale) indicate that K. × houghtonii could expand largely on Mediterranean and subtropical regions of the planet; (3) the HF variable included in the modelling showed strikingly high contribution weights (even with scores above 50%), highlighting its close relationship with anthropic environments; and (4) a general reduction of the potential range of K. × houghtonii for the year 2070 can be anticipated. Considering the strong association with HF shown by K. × houghtonii, particular attention should be paid to the highly humanized regions to control potential invasion progress of this plant. Finally, this study paves the way to future research aiming to understand whether the hybridization process broaden the ecological niche of K. × houghtonii compared to its parental species.
Source: Ecology - nature.com



