In the cohort described in this study we observed directional changes in both HMO concentrations in breast milk and in infant faces, and in the composition of infant faecal microbiota. HMO concentrations differed between individuals and depended on lactation time and infant age. Earlier studies showed that faecal microbiota structure is highly dynamic during infancy and is associated with multiple factors, including diet20,21,22,23. The variation in the faecal microbiota of infants in our study could be explained by infant age, sex, place and mode of delivery and certain milk HMOs, namely 6′SL, 2′FL, 3FL, 3′SL, LNDFH II (p < 0.05). The effect of factors varied with age. Mode of delivery and breastmilk LNFP III concentrations showed a significant association with faecal microbiota structure at two weeks of age. The important role of the mode of delivery in the initial seeding of the GI tract has been previously reported24,25 and has been linked to various health outcomes, both in infancy and beyond26,27. At six weeks the significant effect of the mode of delivery could still be detected in our data, and also infant sex and milk 3′SL seemed to play a role. A recent study using animal models showed that sex specific, microbiota-independent differences in immunity may lead to the selection of a sex-specific GI microbiota in adult germ free mice28. Sex-related differences in faecal microbiota have been also reported in adults29, in pre-term30, and term infants31, yet the timing and the possible mechanisms that might underlie the sex differentiation of GI microbiota in healthy full term infants remain largely unknown. As male infants tend to have a higher daily milk intake, it is possible that they receive higher doses of microbial and HMO components from the mother’s milk32. At 12 weeks of age, infant sex and LNH were significantly associated with microbiota, however, the significant association between microbiota composition and mode of delivery was no longer present. With the accumulating evidence linking mode of delivery with various health effects later in life26,27,33, it is likely that the microbiota related programming of the host happens soon after birth, or during specific “windows of opportunity”, thus, even when microbiota recovers to its normal state, the long term health effects of such disturbance might persist throughout life33.
One of the crucial factors shaping the development of GI microbial community during infancy is the type of feeding that an infant receives, with formula- and breastfed infants showing very different microbial profiles34,35,36,37,38. Human milk is a source of prebiotic HMOs which support microbial colonisation in the infant GI tract7,16,39. The HMO content in breastmilk is variable and influenced by maternal, environmental and infant feeding practices12. The HMO content is highest in colostrum, and the concentrations of HMOs decrease in mature milk8,9,10. Our data agrees with these findings showing that concentrations of the HMOs measured per mL of milk varied between mothers, and decreased between two and 12 weeks of lactation, except for 3FL and LNFP III, which increased in concentration as lactation progressed. Earlier studies showed that the daily intake of milk increases in the first months of life19 likely compensating for the decreased HMO concentrations. Considering the daily milk intake, we could conclude that in our study the amounts of ingested HMOs remained relatively stable during the first 12 weeks of life. The exception was in the intake of 3FL and LNFP III which gradually increased in time. Interestingly, this increase in the 3FL and LNFP III intake corresponded with the increase in the faecal concentrations of these two HMOs, suggesting that, on average, their supply likely exceeded the ability of the infant GI tract microbiota to utilise these two HMOs. Furthermore, the role of these structures might go beyond their prebiotic effect, as both 3FL and LNFP III can act as decoys against pathogen binding and infection40,41,42.
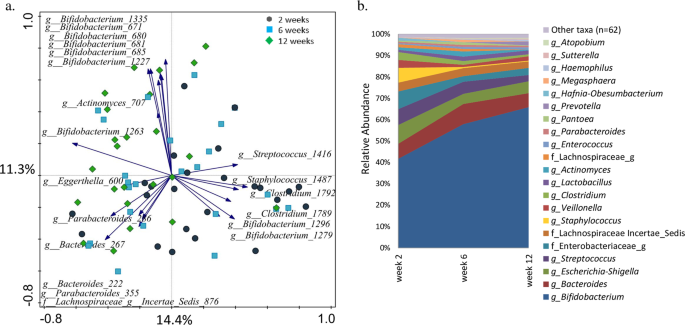
One of the signature bacterial groups found in faeces of breastfed infants is the genus Bifidobacterium2. In vitro studies showed that Bifidobacterium bifidum JCM125439, Bifidobacterium longum subsp. infantis, Bacteroides fragilis and Bacteroides vulgatus16 grow well on HMOs as sole carbon source. Thus, we expected to find positive correlations between certain breastmilk HMOs and the bifidobacterial OTUs. We did not measure the absolute abundance of bacteria in infant faeces and are aware of the limitation of using relative abundance data. However, culture-based studies showed that the total faecal bacterial load, as well as Bifidobacterium counts tend to increase in the first month of life35. Thus, the observed increase in the number of sequencing reads and the relative abundance of bifidobacteria in our data likely reflect the actual increase in the abundance of this group (Fig. S1). We hypothesized that quantity of HMOs might selectively promote growth of either the primary or secondary HMO degraders, leading to increase in their abundance within the microbial community. However, when using data from the three time points combined, we saw an opposite trend – as the predicted daily intake of most HMOs was stable in time, the relative abundance of bifidobacteria was increasing. The analyses repeated for individual time points showed few positive correlations and the results varied between time points (Fig. S3a–c). This could be due to the fact that, in addition to individual HMOs promoting growth of selected microbes capable to utilise this carbon source, there may be other mechanisms controlling the microbial community structure. For example, presence of other breastmilk components, such as lysozyme, secretory IgA and other endogenous factors can suppress growth of certain members of the community and thus, indirectly allow other bacterial species to dominate the infant GI tract ecosystem35. Furthermore, the possible effect of HMOs on microbiota may already start in the breastmilk itself43. A recent study on human milk investigated the associations between the HMO content and microbiota composition in colostrum and reported strong positive correlations between different HMOs and various microbial groups, including streptococci, staphylococci, enterococci and bifidobacteria, in particular Bifidobacterium breve and LNFP III44. In the present study, RDA showed a significant association of milk LNFP III with infant faecal microbiota in all time points combined. There was a strong positive association of milk LNFP III with OTUs belonging to the genera Veillonella, Enterococcus and Streptococcus (Fig. S3). Positive associations at all time points for breastmilk 3′SL and unidentified OTUs within family Enterobacteriaceae and Actinomyces 695 were also observed. 6′SL was positively associated with clostridia – Clostridium 1789 at week two and Clostridium 1639 at week 12. Finally, LNFP III was positively associated with Enterococcus 1698 at six and 12 weeks. At two and at six weeks Lachnospiraceae Incertae Sedis 876 was negatively associated with LNDFH II, Lactobacillus 1718 was negatively associated with 3′SL, and Bifidobacterium 1295 with LNFP I. Studies on mature breastmilk microbiota and the microbial transfer of microbiota from mother to infant show that breastmilk contains a distinct microbial community and that breastfed infants receive on average nearly 30% of the bacteria from breastmilk and 10% from areolar skin in the first 30 days of life45. The study also concluded that the association was lower in older infants, and it was proportional to the frequency of breastfeeding that an infant received45. Thus, it is then likely that some of the correlations observed here were due to a combined effect of the HMOs modulating the microbiota of the mother’s milk and the infant GI tract, as well as due to a direct transfer of bacteria during breastfeeding43.
Infant GI microbiota plays an important role in energy metabolism via utilising otherwise indigestible HMOs. Our data showed that the average concentrations of faecal HMOs decreased with age, suggesting that microbiota of older infants was more adapted and efficient in degrading these compounds (Fig. 5b). Furthermore, we noted that the increase in efficiency was correlated with the increase in the relative abundance of several bifidobacterial OTUs, but also Parabacteroides, Escherichia-Shigella, Bacteroides, Actinomyces, Veillonella, and Erysipelotrichaceae Incertae Sedis (Fig. 6). High ability to degrade a wide range of HMOs was associated with higher relative abundance of one or more Bifidobacterium OTUs confirming the important role of this bacterial group in the HMO metabolism (Fig. 6). Thirteen faecal HMOs negatively correlated with nine different Bifidobacterium OTUs, and the highly abundant Bifidobacterium OTU 1263 was negatively correlated with nine different HMOs in faeces, especially LNH, LNT and LNnT and LNFP V. Aside of bifidobacteria, members of the genus Bacteroides were significantly more abundant in infants who were good degraders of 2′FL, LNFP I, II, V, and pLNH, and Parabacteroides in the high degraders of 3FL, LNFP V, LNH, LNT and LNnT, indicating that these microbial groups might have a mutualistic or symbiotic relationship degrading those compounds. In addition, Halomonas, Enterococcus, Lactobacillus, Staphylococcus, Suterella, Varibaculum, Veillonella, Streptococcus, Actinomyces, and Lachnospiraceae Incertae Sedis were also associated with degradation of the same HMOs as bifidobacteria. Interestingly, high levels of degradation of 6′SL, DFL, LST a and LST c were not associated with high levels of any of the bifidobacterial OTUs, but with various OTUs belonging to Bacteroides, Streptococcus, Varibacullum (6′SL), Actinomyces, Clostridium, Collinsella and Streptococcus (LST c), and Haemophilus, Veillonella (DFL), and Lachnospiraceae Incertae Sedis, and Halomonas (LST a).
Negative associations were also observed for LNFP II and Bacteroides, and for LNFP V and Parabacteroides suggesting the role of these bacteria in the HMO degradation. The fact that Bacteroides and Parabacteroides (formerly also Bacteroides) were identified in our analysis is in line with earlier studies showing growth of Bacteroides spp. on selected milk glycans by activating the mucus degradation pathway46. Finally, LNDFH I in both milk and faeces was negatively associated with Enterococcus OTU 1702, but the association was stronger in faeces. Even though in vitro studies showed that in a monoculture Enterococcus was not able to grow on milk HMOs16, another study showed that this group was found in breastmilk44, that its abundance in infant faeces could be predicted from the maternal HMO profile and that it was positively correlated with the abundance of Bifidobacterium, Streptococcus and Veillonella7. One of the suggested explanations was that Enterococcus can cross feed on HMO fermentation products or HMO breakdown by-products that are released in the ecosystem by HMO degrading bifidobacteria or Bacteroides spp.7.
The correlation analysis of infant faecal HMOs and infant faecal OTUs for all time points combined also detected numerous significant positive associations between various HMOs and Streptococcus, Staphylococcus, Escherichia-Shigella, and Clostridium OTUs (Fig. 5). In both, milk and faeces LST c, 6′SL and LNnH showed significant correlation with staphylococci, while LNFP III and 3FL were positively correlated with streptococci. Earlier studies showed that neither Streptococcus, Staphylococcus, Escherichia-Shigella7,16, nor Clostridium16 could effectively utilise and grow on milk HMOs. However, all these bacterial groups are members of the microbiota of breastmilk and areolar skin45,47,48, and the positive link might be due to breastfeeding practises, for example with more frequent feedings resulting in higher ingested doses of the bacteria and the HMOs. If the HMOs are not well digested, the positive associations may still persist in the faeces.
Our study has few important limitations. The number of participating mother-infant pairs was relatively small and future studies should be done on larger sample sizes to verify our findings. Larger sample size would also allow for stratification of data based on maternal secretor status. Another limitation of this study is the lack of measurements of the actual volumes of the breast milk ingested and the mass of the faeces produced by each infant in a 24-hour sampling period. Therefore, our calculations had to be based on estimates from other studies that measured the daily milk intake in infants of similar ages. Actual intakes, although likely increasing over time, can vary greatly between infants, and thus, our calculated values are only an estimate and should therefore be considered as such. Measurements of volumes or weights of breastmilk samples and faecal samples should be included in future studies. Finally, with our methods we could not identify species or strains of the bacteria, nor could we quantify their actual numbers which could further support our findings.
In this study we found only weak associations between selected breastmilk HMOs and faecal microbiota community structure in breastfed infants. However, we found a strong link between changes in levels of various HMOs in infant faeces and specific microbial groups, in particular different species of Bifidobacterium. Earlier studies showed that different bifidobacterial species vary in their ability to break down HMOs, and some species can degrade HMOs without experiencing a detectable population growth49,50. Thus, including metatranscriptome or metaproteome analyses in this set would have been very helpful in understanding the community dynamics in regard of HMO metabolism in the infant GI tract. Our findings could provide the basis for assembling simple synthetic communities to study microbial interactions and community structure changes, which are centred around degradation of different HMO structures. In vitro fermentation studies incorporating purified compounds would also help to eliminate confounders, such as presence of milk’s own microbiota and presence of milk components, which have a regulatory effect on microbiota in both, milk and in the infant GI tract.
Source: Ecology - nature.com



