Bender, E. A., Case, T. J. & Gilpin, M. E. Perturbation experiments in community ecology: Theory and practice. Ecology 65, 1–13 (1984).
Novak, M. et al. Characterizing species interactions to understand press perturbations: What is the community matrix? Annual Review of Ecology, Evolution, and Systematics 47, 409–432 (2016).
Hobbs, R. J. & Huenneke, L. F. Disturbance, diversity, and invasion: implications for conservation. Conservation Biology 6, 324–337 (1992).
Cayuela, L., Golicher, D. J., Benayas, J. M. R., González-Espinosa, M. & Ramírez-Marcial, N. Fragmentation, disturbance and tree diversity conservation in tropical montane forests. Journal of Applied Ecology 43, 1172–1181 (2006).
Clavel, J., Julliard, R. & Devictor, V. Worldwide decline of specialist species: toward a global functional homogenization? Frontiers in Ecology and the Environment 9, 222–228 (2011).
Socolar, J. B., Gilroy, J. J., Kunin, W. E. & Edwards, D. P. How should beta-diversity inform biodiversity conservation? Trends in Ecology and Evolution 31, 67–80 (2016).
Dornelas, M. et al. Assemblage time series reveal biodiversity change but not systematic loss. Science 344, 296–299 (2014).
McGill, B. J., Dornelas, M., Gotelli, N. J. & Magurran, A. E. Fifteen forms of biodiversity trend in the Anthropocene. Trends in Ecology and Evolution 30, 104–113 (2015).
Anderson, M. J. et al. Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecology Letters 14, 19–28 (2011).
McKinney, M. L. & Lockwood, J. L. Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends in Ecology and Evolution 14, 450–453 (1999).
Olden, J. D. & Poff, N. L. Ecological processes driving biotic homogenization: testing a mechanistic model using fish faunas. Ecology 85, 1867–1875 (2004).
Karp, D. S., Rominger, A. J., Zook, J. & Ranganathan, J. & others. Intensive agriculture erodes β‐diversity at large scales. Ecology 15, 963–970 (2012).
Iacarella, J. C. et al. Anthropogenic disturbance homogenizes seagrass fish communities. Global Change Biology 24, 1904–1918 (2018).
Zaneveld, J. R. et al. Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales. Nature Communications 7, 11833 (2016).
McDevitt-Irwin, J. M., Baum, J. K., Garren, M. & Vega Thurber, R. L. Responses of coral-associated bacterial communities to local and global stressors. Frontiers in Marine Science 4, 262 (2017).
McDevitt-Irwin, J. M., Garren, M., McMinds, R., Vega Thurber, R. & Baum, J. K. Variable interaction outcomes of local disturbance and El Niño-induced heat stress on coral microbiome alpha and beta diversity. Coral Reefs early onli, (2019).
Zaneveld, J. R., McMinds, R. & Vega Thurber, R. Stress and stability: applying the Anna Karenina principle to animal microbiomes. Nature Microbiology 2, 17121 (2017).
Wright, R. M. et al. Intraspecific differences in molecular stress responses and coral pathobiome contribute to mortality under bacterial challenge in Acropora millepora. Scientific Reports 7 (2017).
Quigley, K. M., Willis, B. L. & Bay, L. K. Maternal effects and Symbiodinium community composition drive differential patterns in juvenile survival in the coral Acropora tenuis. Royal Society Open Science 3, 160471 (2016).
Bellwood, D. R., Hughes, T. P., Folke, C. & Nyström, M. Confronting the coral reef crisis. Nature 429, 827–833 (2004).
LaJeunesse, T. C. et al. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Current Biology 28, 2570–2580 (2018).
Wiedenmann, J. et al. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nature Climate Change 3, 160 (2012).
Cunning, R. & Baker, A. C. Excess algal symbionts increase the susceptibility of reef corals to bleaching. Nature Climate Change 3, 259–262 (2013).
Vega Thurber, R. L. et al. Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Global Change Biology 20, 544–554 (2014).
Brown, B. E. Coral bleaching: causes and consequences. Coral Reefs 16, S129–S138 (1997).
Stat, M., Morris, E. & Gates, R. D. Functional diversity in coral-dinoflagellate symbiosis. Proceedings of the National Academy of Sciences 105, 9256–9261 (2008).
Suggett, D. J., Warner, M. E. & Leggat, W. Symbiotic dinoflagellate functional diversity mediates coral survival under ecological crisis. Trends in Ecology & Evolution 32, 735–745 (2017).
Quigley, K. M., Bay, L. K. & Willis, B. L. Temperature and water quality-related patterns in sediment-associated Symbiodinium communities impact symbiont uptake and fitness of juveniles in the genus Acropora. Frontiers in Marine Science 4, 401 (2017).
Tonk, L., Bongaerts, P., Sampayo, E. M. & Hoegh-Guldberg, O. SymbioGBR: a web-based database of Symbiodinium associated with cnidarian hosts on the Great Barrier Reef. BMC Ecology 13, 7 (2013).
Kennedy, E. V. et al. Symbiodinium biogeography tracks environmental patterns rather than host genetics in a key caribbean reef-builder, Orbicella annularis. Proceedings of the Royal Society B: Biological Sciences 283 (2016).
Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Marine and Freshwater Research 50, 839–866 (1999).
Hughes, T. P. et al. Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933 (2003).
Hughes, T. P. et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83 (2018).
Richmond, R. H. & Hunter, C. L. Reproduction and recruitment of corals: comparisons among the Caribbean, the Tropical Pacific, and the Red Sea. Marine Ecology Progress Series 60, 185–203 (1990).
Baird, A. H., Guest, J. R. & Willis, B. L. Systematic and biogeographical patterns in the reproductive biology of scleractinian sorals. Annual Review of Ecology, Evolution, and Systematics 40, 551–571 (2009).
Hartmann, A. C. et al. Acquisition of obligate mutualist symbionts during the larval stage is not beneficial for a coral host. Molecular Ecology 28, 141–155 (2019).
LaJeunesse, T. C. et al. Host-symbiont recombination versus natural selection in the response of coral-dinoflagellate symbioses to environmental disturbance. Proceedings of the Royal Society B: Biological Sciences 277, 2925–2934 (2010).
Boulotte, N. M. et al. Exploring the Symbiodinium rare biosphere provides evidence for symbiont switching in reef-building corals. The ISME Journal 10, 2693–2701 (2016).
Little, A. F., van Oppen, M. J. H. & Willis, B. L. Flexibility in algal endosymbioses shapes growth in reef corals. Science 304, 1492–1494 (2004).
Coffroth, M. A., Poland, D. M., Petrou, E. L., Brazeau, D. A. & Holmberg, J. C. Environmental symbiont acquisition may not be the solution to warming seas for reef-building corals. PLoS ONE 5, e13258 (2010).
Manning, M. M. & Gates, R. D. Diversity in populations of free-living Symbiodinium from a Caribbean and Pacific reef. Limnology and Oceanography 53, 1853 (2008).
Littman, R. A., van Oppen, M. J. H. & Willis, B. L. Methods for sampling free-living Symbiodinium (zooxanthellae) and their distribution and abundance at Lizard Island (Great Barrier Reef). Journal of Experimental Marine Biology and Ecology 48–53 (2008).
Takabayashi, M., Adams, L. M., Pochon, X. & Gates, R. D. Genetic diversity of free-living Symbiodinium in surface water and sediment of Hawai’i and Florida. Coral Reefs 31, 157–167 (2012).
Granados-Cifuentes, C., Neigel, J., Leberg, P. & Rodriguez-Lanetty, M. Genetic diversity of free-living Symbiodinium in the Caribbean: the importance of habitats and seasons. Coral Reefs 34, 927–939 (2015).
Lee, M. J., Jeong, H. J., Jang, S. H., Lee, S. Y. & Kang, N. S. S. Most low-abundance ‘background’ Symbiodinium spp. are transitory and have minimal functional significance for symbiotic corals. Microbial Ecology 71, 771–83 (2016).
Yamashita, H. & Koike, K. Genetic identity of free-living Symbiodinium obtained over a broad latitudinal range in the Japanese coast. Phycological Research 61, 68–80 (2013).
Nitschke, M. R., Davy, S. K. & Ward, S. Horizontal transmission of Symbiodinium cells between adult and juvenile corals is aided by benthic sediment. Coral Reefs 35, 335–344 (2016).
Thornhill, D. J., Howells, E. J., Wham, D. C., Steury, T. D. & Santos, S. R. Population genetics of reef coral endosymbionts (Symbiodinium, Dinophyceae). Molecular Ecology 26, 2640–2659 (2017).
Pochon, X. et al. Comparison of endosymbiotic and free-living Symbiodinium (Dinophyceae) diversity in a Hawaiian reef environment. Journal of Phycology 46, 53–65 (2010).
Huang, H. et al. Diversity of free-living and symbiotic Symbiodinium in the coral reefs of Sanya, South China Sea. Marine Biology Research 9, 117–128 (2013).
Sweet, M. J. Symbiodinium diversity within Acropora muricata and the surrounding environment. Marine Ecology 35, 343–353 (2014).
Cunning, R., Yost, D. M., Guarinello, M. L., Putnam, H. M. & Gates, R. D. Variability of Symbiodinium communities in waters, sediments, and corals of thermally distinct reef pools in American Samoa. PLoS One 10, e0145099 (2015).
Callahan, B. J., McMurdie, P. J. & Holmes, S. P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. The ISME Journal 11, 2639–2643 (2017).
Lee-Cruz, L., Edwards, D. P., Tripathi, B. M. & Adams, J. M. Impact of logging and forest conversion to oil palm plantations on soil bacterial communities in Borneo. Applied and Environmental Microbiology 79, 7290–7297 (2013).
de Carvalho, T. S. et al. Land use intensification in the humid tropics increased both alpha and beta diversity of soil bacteria. Ecology 97, 2760–2771 (2016).
Cooper, T. F. et al. Environmental factors controlling the distribution of Symbiodinium harboured by the coral Acropora millepora on the Great Barrier Reef. PLoS ONE 6, e25536 (2011).
Putnam, H. M., Stat, M., Pochon, X. & Gates, R. D. Endosymbiotic flexibility associates with environmental sensitivity in scleractinian corals. Proceedings of the Royal Society B: Biological Sciences 279, 4352–4361 (2012).
Cunning, R., Gates, R. D. & Edmunds, P. J. Using high-throughput sequencing of ITS2 to describe Symbiodinium metacommunities in St. John, US Virgin Islands. PeerJ 5, e3472 (2017).
Arif, C. et al. Assessing Symbiodinium diversity in scleractinian corals via next-generation sequencing-based genotyping of the ITS2 rDNA region. Molecular Ecology 23, 4418–4433 (2014).
Quigley, K. M. et al. Deep-sequencing method for quantifying background abundances of Symbiodinium types: exploring the rare Symbiodinium biosphere in reef-building corals. PLoS ONE 9, e94297 (2014).
Green, E. A., Davies, S. W., Matz, M. V. & Medina, M. Quantifying cryptic Symbiodinium diversity within Orbicella faveolata and Orbicella franksi at the Flower Garden Banks, Gulf of Mexico. PeerJ 2, e386 (2014).
Ziegler, M., Stone, E., Colman, D., Takacs-Vesbach, C. & Shepherd, U. Patterns of Symbiodinium (Dinophyceae) diversity and assemblages among diverse hosts and the coral reef environment of Lizard Island, Australia. Journal of Phycology 54, 447–460 (2018).
Treml, E. A., Halpin, P. N., Urban, D. L. & Pratson, L. F. Modeling population connectivity by ocean currents, a graph-theoretic approach for marine conservation. Landscape Ecology 23, 19–36 (2008).
Baums, I. B., Devlin-Durante, M. K. & LaJeunesse, T. C. New insights into the dynamics between reef corals and their associated dinoflagellate endosymbionts from population genetic studies. Molecular Ecology 23, 4203–4204 (2014).
Fitt, W. K. & Trench, R. K. The relation of diel patterns of cell division to diel patterns of motility in the symbiotic dinoflagellate Symbiodinium microadriaticum Freudenthal in culture. New Phytologist 94, 421–432 (1983).
Kirk, N. L., Andras, J. P., Harvell, C. D., Santos, S. R. & Coffroth, M. A. Population structure of Symbiodinium sp. associated with the common sea fan, Gorgonia ventalina, in the Florida Keys across distance, depth, and time. Marine Biology 156, 1609–1623 (2009).
Nitschke, M. R. The free-living Symbiodinium reservoir and scleractinian coral symbiont acquisition. (espace.library.uq.edu.au, 2015).
Harii, S., Yamamoto, M. & Hoegh-Guldberg, O. The relative contribution of dinoflagellate photosynthesis and stored lipids to the survivorship of symbiotic larvae of the reef-building corals. Marine Biology 157, 1215–1224 (2010).
Connolly, S. R. & Baird, A. H. Estimating dispersal potential for marine larvae: dynamic models applied to scleractinian corals. Ecology 91, 3572–3583 (2010).
Graham, E. M., Baird, A. H. & Connolly, S. R. Survival dynamics of scleractinian coral larvae and implications for dispersal. Coral Reefs 27, 529–539 (2008).
Carilli, J. & Walsh, S. Benthic foraminiferal assemblages from Kiritimati (Christmas) Island indicate human-mediated nutrification has occurred over the scale of decades. Marine Ecology Progress Series 456, 87–99 (2012).
Pochon, X. & Pawlowski, J. & Others. Evolution of the soritids-Symbiodinium symbiosis. Symbiosis 42, 77–88 (2006).
Silverstein, R. N., Correa, A. M. S. & Baker, A. C. Specificity is rarely absolute in coral-algal symbiosis: implications for coral response to climate change. Proceedings of the Royal Society B: Biological Sciences 279, 2609–2618 (2012).
Quigley, K. M., Willis, B. L. & Bay, L. K. Heritability of the Symbiodinium community in vertically-and horizontally-transmitting broadcast spawning corals. Scientific Reports 7 (2017).
Bay, L. K., Doyle, J., Logan, M. & Berkelmans, R. Recovery from bleaching is mediated by threshold densities of background thermo-tolerant symbiont types in a reef-building coral. Royal Society Open Science 3, 160322 (2016).
Kojis, B. L. & Quinn, N. J. Reproductive strategies in four species of Porites (Scleractinia). In Proceedings of the 4th International Coral Reef Symposium 145–151 (1981).
Willis, B. L., Babcock, R. C., Harrison, P. L. & Oliver, J. K. Patterns in the mass spawning of corals on the Great Barrier Reef from 1981 to 1984. In Proceedings of the Fifth International Coral Reef Congress, Tahiti, 1985, Vol. 4 343–348 (1985).
Byler, K. A., Carmi-Veal, M., Fine, M. & Goulet, T. L. Multiple symbiont acquisition strategies as an adaptive mechanism in the coral Stylophora pistillata. PLoS ONE 8, e59596 (2013).
Quigley, K. M., Warner, P. A., Bay, L. K. & Willis, B. L. Unexpected mixed-mode transmission and moderate genetic regulation of Symbiodinium communities in a brooding coral. Heredity 121, 524–536 (2018).
Baker, A. C., Starger, C. J., McClanahan, T. R. & Glynn, P. W. Coral reefs: corals’ adaptive response to climate change. Nature 430, 741 (2004).
Rowan, R. Coral bleaching: Thermal adaptation in reef coral symbionts. Nature 430, 742 (2004).
Jones, A. M., Berkelmans, R., van Oppen, M. J. H., Mieog, J. C. & Sinclair, W. A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proceedings of the Royal Society B: Biological Sciences 275, 1359–1365 (2008).
Baker, D. M., Freeman, C. J., Wong, J. C. Y., Fogel, M. L. & Knowlton, N. Climate change promotes parasitism in a coral symbiosis. The ISME Journal 12, 921–930 (2018).
Frank, S. A. Host-symbiont conflict over the mixing of symbiotic lineages. Proceedings of the Royal Society B: Biological Sciences 263 (1996).
Walsh, S. M. Ecosystem-scale effects of nutrients and fishing on coral reefs. Journal of Marine Biology 2011, 1–13 (2011).
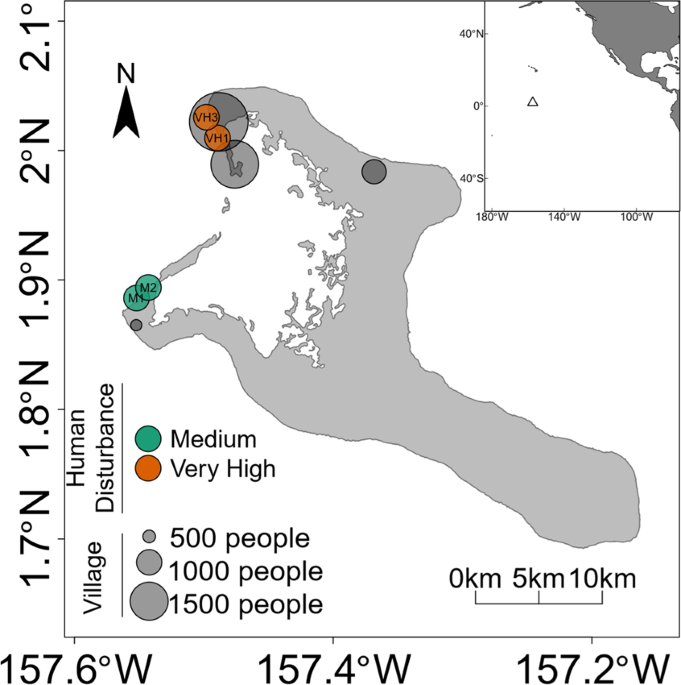
Watson, M. S., Claar, D. C. & Baum, J. K. Subsistence in isolation: Fishing dependence and perceptions of change on Kiritimati, the world’s largest atoll. Ocean and Coastal Management 123, 1–8 (2016).
Morate, O. 2015 Population and Housing Census. Volume 1: Management Report and Basic Tables. (2016).
Tolman, H. & Chalikov, D. Development of a third-generation ocean wave model at NOAA/NCEP. in Int. Sym. Waves: Physical & Numerical Modelling (ed. M. Isaacson, M. C. Q.) 724–733 (University of British Columbia Press, 1994).
Tolman, H. L. & Others. User manual and system documentation of WAVEWATCH III version 3.14. Technical note, MMAB Contribution 276, 220 (2009).
Gilleland, E. & Katz, R. W. & Others. extRemes 2.0: an extreme value analysis package in R. Journal of Statistical Software 72, 1–39 (2016).
Millard, S. P. EnvStats: An R package for environmental statistics. (Springer, 2013).
Stat, M., Loh, W. K. W., LaJeunesse, T. C., Hoegh-Guldberg, O. & Carter, D. A. Stability of coral-endosymbiont associations during and after a thermal stress event in the southern Great Barrier Reef. Coral Reefs 28, 709–713 (2009).
Cunning, R., Silverstein, R. N. & Baker, A. C. Investigating the causes and consequences of symbiont shuffling in a multi-partner reef coral symbiosis under environmental change. Proceedings of the Royal Society B: Biological Sciences 282, 20141725 (2015).
Thornhill, D. J., Lajeunesse, T. C. & Santos, S. R. Measuring rDNA diversity in eukaryotic microbial systems: How intragenomic variation, pseudogenes, and PCR artifacts confound biodiversity estimates. Molecular Ecology 16, 5326–5340 (2007).
Smith, E. G., Hume, B. C. C., Delaney, P., Wiedenmann, J. & Burt, J. A. Genetic structure of coral-Symbiodinium symbioses on the world’s warmest reefs. PLoS One 12, e0180169 (2017).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nature Methods 13, 581–583 (2016).
McMurdie, P. J. & Holmes, S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8, e61217 (2013).
R Development Core Team. R: A language and environment for statistical computing (2008).
Wright, E. S. Using DECIPHER v2.0 to analyze big biological sequence data in R. The R Journal 8, 352–359 (2016).
Pochon, X. & Gates, R. D. A new Symbiodinium clade (Dinophyceae) from soritid foraminifera in Hawaii. Molecular Phylogenetics and Evolution 56, 6 (2010).
Schliep, K. P. phangorn: phylogenetic analysis in R. Bioinformatics 27, 592–593 (2011).
Oksanen, J. Vegan: an introduction to ordination (2017).
Lozupone, C., Lladser, M. E., Knights, D., Stombaugh, J. & Knight, R. UniFrac: an effective distance metric for microbial community comparison. The ISME Journal 5, 169–172 (2011).
Anderson, M. J. & Walsh, D. C. I. Permanova, anosim, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecological Monographs 83, 557–574 (2013).
Larsson, J. eulerr: Area-proportional Euler and Venn diagrams with circles or ellipses. Available at: https://cran.r-project.org/package=eulerr (2018).
Chao, A. et al. Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol. Monogr. 84, 45–67 (2014).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis. (Springer, 2016).
Source: Ecology - nature.com



