After excluding data for Elapsoidea because of the small sample size, we were left with data for 77 Bitis nasicornis, 68 Dendroaspis jamesoni, 228 D. polylepis, 36 Naja subfulva and 75 N. annulata.
Rates of capture
The most striking feature of Ionides’ records is the high daily rates of capture. For example, he collected 47 D. polylepis within a two-week period (in October 1965), 34 D. jamesoni over a six-week period (September-October 1961), 25 N. annulata over a two-week period (August 1958), and 23 B. nasicornis within a month (October 1961).
Changes in species captured over the years
The relative numbers of each species collected varied strongly through time (nominal logistic regression with year as factor and species as dependent variable; χ2 = 921.56, df = 76, p < 0.0001). All of the early records (from 1945 to 1953) concern a single taxon (D. polylepis). In contrast, N. annulata and D. jamesoni each were taken in only three years (respectively, 1956, 1958, 1960; and 1959, 1961, and 1963). Bitis nasicornis was collected in 1954, 1959, 1961 and 1963. Naja subfulva was taken at lower rates over a longer period (1954 to 1967; N = 0 to 9 snakes per year).
Capture month
The monthly distribution of captures differed among species (χ2 = 304.22, df = 33, p < 0.0001), but the primary capture period was August to October for all taxa (Fig. 3). All records of B. nasicornis and D. jamesoni fall within these three months, as do 81% of captures of D. polylepis and 67% of N. subfulva. In contrast, N. annulata were taken primarily in July (48%) and August (44%).
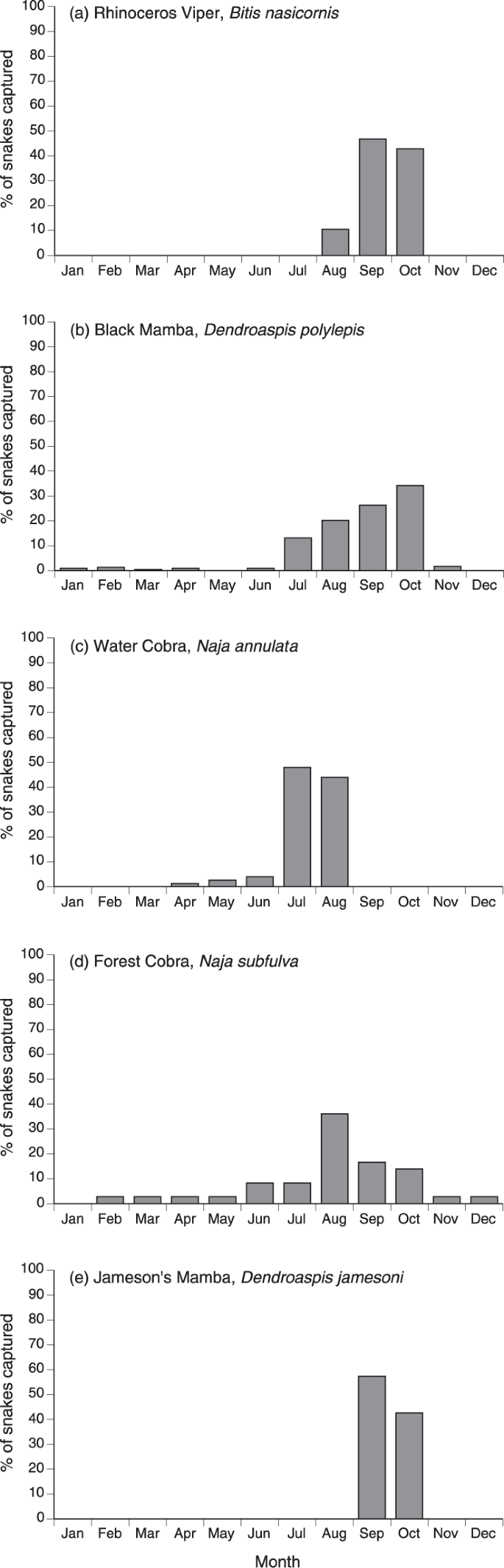
Monthly distribution of capture records for five species of snakes in east Africa, from the notebooks of C.J.P. Ionides.
Capture month vs. life stage
In a two-factor ANOVA with species and life stage as factors, the interaction between these two variables significantly affected month of collection (F15,451 = 1.82, p < 0.03). However, no relationship between life stage and month of capture was statistically significant when analyses were conducted separately for each species.
Capture month vs. sex
Sex ratios of captured snakes did not differ significantly among months in the overall sample, but monthly variation was significant within N. subfulva (F10,35 = 19.50, p < 0.035). However, the divergence between sexes was minor (female-bias in September, male-bias in October) and unlikely to be biologically significant.
Capture time of day
Except for 10 snakes that Ionides recorded as being captured in the evening (without specific times), all snakes were taken between 0730 h and 1900 h. There were no strong peaks or troughs within that period, but capture times differed among species. The relative numbers of captures in the morning (versus the afternoon) was 56% for D. jamesoni, 46.5% for D. polylepis, 34.2% for B. nasicornis, 30.6% for N. subfulva, and 20% for N. annulata. Those interspecific divergences are statistically significant (χ2 = 27.03, df = 4, p < 0.0001).
Capture time of day vs. life stage
In a nominal logistic regression, capture time (AM vs. PM) was affected both by species (χ2 = 29.79, df = 4, p < 0.0001) and by life stage (χ2 = 7.11, df = 1, p < 0.008; interaction term NS so deleted). Figure 4 depicts the effect of life stage on time of capture, with a higher proportion of juveniles captured in the morning and older age classes captured in the afternoon.
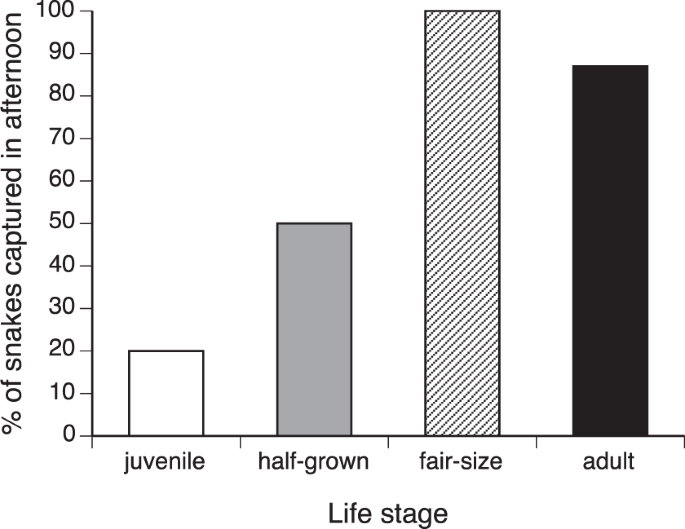
Times of day at which C.J.P. Ionides captured different age classes of snakes of five species. Data are combined for five species, because statistical analysis revealed no significant interspecific differences in these patterns (see text). The histograms show the proportion of total captures for each age class that were caught in the afternoon rather than the morning. Most captures of juvenile snakes were in the morning, whereas larger snakes were generally captured in the afternoon.
Species differences in relative numbers of each life stage
Ionides’ samples of all species were dominated by adults (from 61% in B. nasicornis to 87% in D. jamesoni), but the proportion of juvenile animals was highest in N. subfulva (19%) and lowest in D. polylepis (4%; treating life stage as an ordinal variable, species effect F4,477 = 3.62, p < 0.007).
Sex ratio
Sex ratios of the collected snakes varied among species (logistic regression – χ2 = 16.12, df = 4, p < 0.003), being female-biased in B. nasicornis (59.7%) and N. annulata (62.7%) but male-biased in the terrestrial elapids (D. polylepis 40.8% female, D. jamesoni 47.1%, N. subfulva 41.7%).
If we include life stage as well as species as factors in a nominal logistic regression, sex ratios are influenced by both of these variables (species effect χ2 = 16.09, df = 4, p < 0.003; life stage effect χ2 = 6.60, df = 2, p < 0.015; interaction NS so deleted; see Fig. 5). Samples of younger snakes were female-biased (62.5% females in juveniles, 67.6% in half-grown animals) whereas most larger snakes were males (45.0% female in fair-sized animals, 45.4% in adults). The trend was also statistically significant within N. subfulva (χ2 = 6.48, df = 3, p < 0.011).
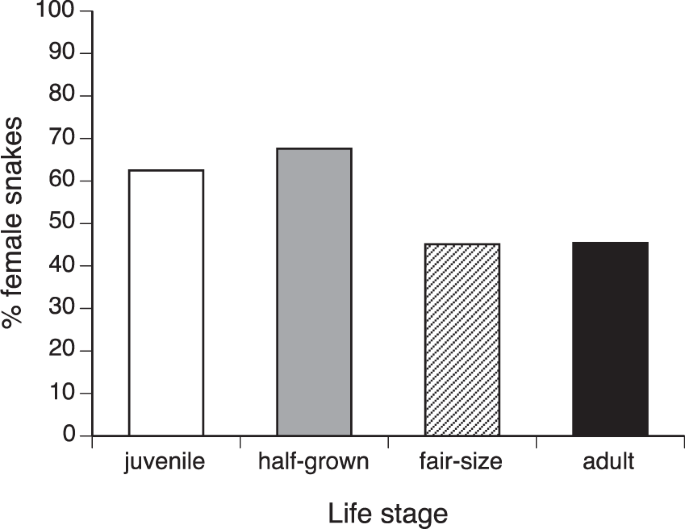
Ontogenetic (age-class) variation in sex ratios of snakes captured by C.J.P. Ionides. Data are combined for five species, because statistical analysis revealed no significant interspecific differences in these patterns (see text). The proportion of snakes that were females declined among larger size classes.
Habitat use
For analysis, we divided habitats into four categories (arboreal, terrestrial, below-ground, in water). The species differed in terms of relative numbers within each of these categories (χ2 = 450.70, df = 12, p < 0.0001; interaction between species and life stage χ2 = 20.87, df = 12, p = 0.052). The species effect reflects a pattern in which the two mamba species were found primarily in trees, the water cobra was found in the water, the rhinoceros viper was found both on the ground and in trees, and the eastern forest cobra was found in all habitats. Looking separately within each species, the ontogenetic shift in habitat use was statistically significant within B. nasicornis (shift from arboreal to terrestrial sites with increasing age; χ2 = 8.73, df = 2, p < 0.015) and D. polylepis (adult snakes more often found in fossorial rather than arboreal sites; χ2 = 7.13, df = 2, p < 0.03; see Fig. 6).
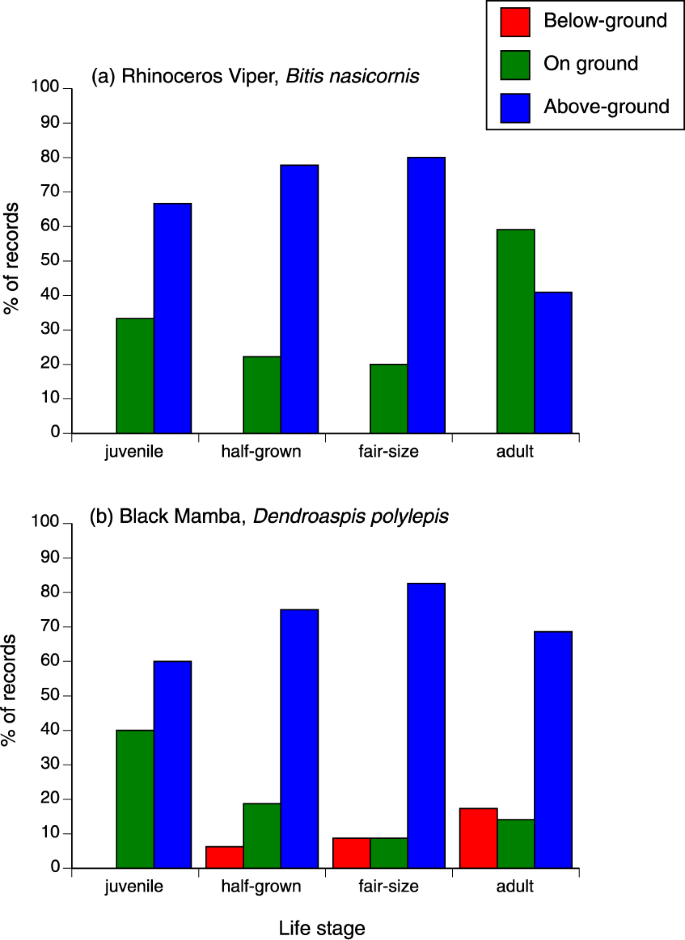
Ontogenetic (age-class) variation in habitat use of rhinoceros vipers Bitis nasicornis, and black mambas Dendroaspis polylepis, captured by C.J.P. Ionides. Arboreality was less common in larger rhinoceros vipers, and fossoriality was more common in larger black mambas.
A snake’s sex also affected its habitat choice (nominal logistic regression; species effect χ2 = 439.19, df = 12, p < 0.0001; sex effect χ2 = 8.88, df = 3, p < 0.035; interaction species*sex NS). The sex difference involved males being found in trees more often than were females (65.4 vs. 51.5%) and also, being found less often on the ground (21.9 vs. 12.5%).
For one species (B. nasicornis), Ionides also recorded height above the ground. No significant differences in mean height above ground were evident as a function of a snake’s sex (F1,21 = 0.79, p = 0.38) or its life stage (F1,21 = 2.64, p = 0.12; and interaction sex*life stage = NS).
Co-occurrence of snakes
Records of snakes found together in the same site may reflect social interactions such as courtship, mating and male-male combat. For D. polylepis, Ionides recorded cases involving two adult males collected from the same antbear hole (October 1965), close to each other within a thicket (October 1959), and smoked out of the same tree (7 days apart, September 1967). A female was captured six days after a male was caught in the same tree (September 1965), a juvenile female and an adult male were both taken from under bark of the same dead tree (October 1965), and an adult male and female were taken from the same antbear hole (October 1965). For Dendroaspis jamesoni, Ionides recorded an adult male plus an adult female in the same trees on four occasions (October 1959, September 1961, October 1961, October 1963), and one additional case of two females (one adult, one fair-sized) in the same tree (October 1963). Lastly, two adult male Naja subfulva were dug out of the same termite mound two days apart in October 1963.
Source: Ecology - nature.com



