Experimental overview
An in situ field experiment was conducted over two years to examine the influence of larval density on initial settlement and post-settlement persistence, coral abundance and growth. Using a regression design, we tested 30 different larval densities of Acropora tenuis in a degraded reef environment. Larvae were initially confined within 30 individual flow-through enclosures each containing a pre-conditioned settlement tile, during the settlement period34,40. The containers were then removed and the tiles and settled spat exposed to the open reef environment. The branching coral species, A. tenuis, was selected due to its ecological significance within the study region, and because spawned gametes and larvae were readily available18.
Site location and preparation
This study was conducted at Magsaysay Reef in the Lingayen Gulf, Anda, Pangasinan, Northern Luzon, the Philippines (16°19′36″N, 120°02′01″E). The reef site was characterised by low mean live scleractinian coral cover (~15%) and high mean cover of macroalgae, turf communities, sponges and soft corals (~57%)18, and encircled a small sand patch at 2–3 m depth.
Coral settlement and persistence of juvenile colonies were monitored on biologically conditioned natural settlement tiles. To mimic natural reef substratum as closely as possible while still allowing for periodic ex situ monitoring under microscopes, 30 tiles were cut from the skeletons of dead tabulate Acropora species collected from a nearby rubble zone18. The irregular surfaces provided a range of natural microhabitats, and each tile was fixed 0.5–2 cm above the substratum to provide the full range of surface orientation available on a natural reef, i.e., exposed upper surfaces, vertical surfaces, and shaded downward facing surfaces (see Supplementary Fig. S1). While there was some natural variation between tiles, average dimensions were 10 × 10 × 2.5 cm (~300 cm−2 surface area). Prior to the experiment, settlement tiles were first conditioned for 6 weeks in aquaculture tanks with flow-through seawater and aeration to acquire initial biofilms at the Bolinao Marine Laboratory (BML), of the University of the Philippines Marine Science Institute. Then on 30 March 2017 the tiles were transferred to the reef site at Magsaysay Reef for an additional ~8 weeks conditioning to further develop biofilms in situ. Tiles were attached to a threaded steel rod projecting from a stainless steel plate fixed directly to the substratum41 (see Supplementary Fig. S1). A coded stainless steel tag was attached to each tile and stainless steel plate, to ensure tiles could be returned to the same location and relocated on the steel posts in the same orientation after monitoring.
Gamete collection and larval culture
Coral cover at Magsaysay Reef is very low, however there are several discrete patches where colonies of sexually mature A. tenuis are present, following previous larval restoration trials18. These patches are approximately 50 m from the experimental site for this study. Field sampling of the A. tenuis colonies on 5 May 2017 revealed dark pink mature eggs, indicating imminent spawning42, with spawning occurring during a fierce electrical storm between 18:30 h and 19:00 h on 10 May 2017, the night of the full moon. During this time, spawn collection nets made from 150 μm plankton mesh were placed over 30 spawning colonies by divers (see Supplementary Fig. S2). Spawned egg-sperm bundles were positively buoyant and collected in a jar attached to the top of each net. Jars were sealed in situ after spawning and gametes from each colony were kept separated during transport to BML, where all gametes were transferred into a large polyethylene container (60 L Nally bin) with ~40 L of 1 µm filtered seawater for fertilisation. Contribution to the larval pool from each colony was roughly equivalent as netted colonies were similar in size and most of the spawned gamete bundles were collected from each colony.
Fertilisation and larval culture followed standard methods18,43. Gamete bundles were gently agitated to facilitate separation of sperm and eggs to maximise cross-fertilisation44. After one hour, the buoyant eggs were carefully siphoned off and placed in a fresh container of filtered seawater, to remove excess sperm. This process was conducted three times, to prevent polyspermy and maintain water quality during larval culture44. The developing embryos were then transferred to 1,000 L rearing tanks and left undisturbed, with ~300,000 embryos in each tank (~0.3 larvae/mL). Prior to transfer to the rearing rearing tanks, five 100 mL subsamples of embryos were collected for examination under dissecting microscopes and it was determined that >90% of the eggs were fertilised. After 24 hours, gentle aeration was supplied and 50 L of new filtered seawater was added daily to replace water lost to evaporation and to help maintain water quality17.
Effect of larval density on settlement and persistence of corals on tiles
To test the effect of larval density on settlement rates and colony persistence, single replicates of 30 larval densities ranging between 10–5,000 were selected for investigation. Previous testing of densities with 500–1,000 larvae per tile indicated settlement rates can range between 10–20%, consistent with 10–15% settlement observed in other in situ settlement studies21. We therefore chose ~5,000 larvae as the upper density limit in this study, assuming this would result in a maximum of ~1,000 initial settlers on the highest density tile (~3.3 settlers/cm2), which would quickly be subject to intraspecific competition22. Early in the morning on the 4th day after spawning (14 May 2017) competent larvae were carefully filtered out of the larval culture tanks with a 60 µm plankton mesh sieve, then extracted with pipettes and counted in Bogorov trays under dissecting microscopes illuminated with LED cold light sources. Groups of larvae that were actively swimming and exhibiting searching behaviour were counted and placed into 30 plastic bowls each containing ~500 mL of filtered seawater corresponding with the preselected larval densities. Manual counting time was minimised to avoid compromising larval health in the concentrated high density samples, so the preselected densities were used as an approximate guide, and where the numbers of larvae counted differed slightly from the predetermined densities, the actual numbers were recorded and used in all subsequent analysis (see Supplementary Dataset 1). A total of 45,518 larvae were individually counted, with the highest density treatment containing 5,130 larvae. Larvae in the bowls were gently swirled every 1–2 hours to prevent early settlement behaviour. Shortly before transfer to the field, larvae were further concentrated into 30 labelled 60 mL syringes, each containing 60 mL of filtered seawater.
The sealed larval syringes were carefully transported from BML to Magsaysay Reef in a large plastic container filled with seawater to regulate temperature and provide gentle agitation to prevent larvae from settling in the syringes. Before release of larvae into settlement enclosures, each in situ tile was briefly removed from its post and visually checked for recent wild coral recruits. A small number of juvenile pocilloporid recruits were observed and removed, however no juvenile Acropora spp. recruits were found. Modified 2.3 L food quality polyethylene containers were used as the settlement containers for the field settlement experiment (Fig. 1a). Each container was threaded over a steel post, with 150 µm plankton mesh windows on each side to allow water exchange while containing the A. tenuis larvae, as these typically exceed 300 µm in diameter18,45. The settlement tile was replaced atop a rubber spacer to seal the post hole in the bottom of the container and the container lid was secured with an O-ring, creating a flow-through container around each tile, with the tile orientated in the same aspect in which it had been conditioning. Each container lid was pre-drilled with a small hole through which the counted larvae were injected (Fig. 1a), then the hole was sealed with a self-tapping screw.
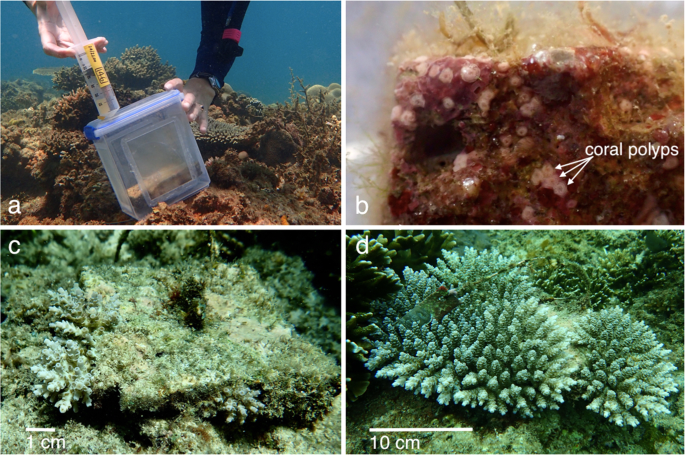
Images from the field site at Magsaysay Reef, Luzon, the Philippines: (a) injecting A. tenuis larvae into the flow-through containers at the Magsaysay Reef site with settlement tile visible within the container, (b) five day old spat on 18 May 2017, visible as small pink polyps, on a settlement tile, (c) juvenile corals surviving after 12 months (5 May 2018) on a tile initally supplied with 991 larvae, and (d) the same tile with surviving juvenile corals after 24 months (24 April 2019). Photos: (a) D. dela Cruz, (b–d) K. Cameron.
The site was monitored daily for five days and all larval containers remained secure. After the five day settlement period, the larval containers were removed and tiles were carefully transported to a nearby field laboratory for examination under dissecting microscopes and LED lights, with the tiles remaining submerged in seawater in large containers. Tiles were initially examined under low magnification to confirm that no previously settled natural Acropora spp. recruits were present. None were detected, consistent with negligible recruitment observed for wild Acropora spat at Magsaysay Reef18. The position on the tile of each newly settled and metamorphosed coral spat was then recorded and scored as either top (upward facing surface), vertical edge, underhang (outer one cm of the downward facing surface) or inner bottom (inner eight cm of the downward facing surface). Each tile surface was also photographed (Fig. 1b). A thick, anoxic film of cyanobacteria had developed on the bottom and sides of the tile with the fourth highest larval density (2978 larvae supplied), rendering it unsuitable for any settlement, so data from this tile were discarded from analyses. The remaining 29 tiles were returned to the study site within several hours after collection, each to the same location and reattached onto the post in the same orientation as it was placed during conditioning and larval settlement.
Tiles were collected and surviving juvenile corals counted again under microscopes after two months (26 July 2017), five months (25 October 2017) and eight months (23 January 2018). At 12 months (5 May 2018, Fig. 1c) and 24 months (24 April 2019, Fig. 1d) the surviving corals were too large for tiles to be removed without damaging the coral colonies, so visual monitoring was completed by divers in the field. Counting individual colonies was challenging after 12 months, as fusing colonies became difficult to distinguish independently, so at 12 and 24 months, the greatest diameter (GD) and least diameter (LD) of each discrete colony or discrete collective of fused colonies was measured with calipers. The geometric mean diameter √ (GD × LD) was subsequently calculated for each colony46 or fused colony, and these were summed to derive the total horizontal area of coral growing on each tile.
Statistical analyses
Data analyses were undertaken using the statistical package R, v.3.3.347. Analyses of initial settlement and colony persistence to 12 months were conducted with generalised additive models (GAM) from the package ‘mgcv’48, using negative-binomial variance structure to account for overdispersion evident in the data. For each census period, the GAM included initial larval supply as the continuous predictor and the number of spat (5 days) or colonies present (2, 5, 8 and 12 months) as the response variable. Analysis of initial settlement rate was conducted with a GAM using binomial variance structure, with initial larval supply as the continuous predictor and the proportion of larvae settled as the response variable. Analysis of coral cover at 12 and 24 months was conducted with GAMs using normal variance structure, with initial larval supply as the continuous predictor, and cm2 of coral cover per tile and average colony size as the response variables. Comparisons of settlement on the available tile surfaces were initially conducted using a multivariate linear model, with initial larval density as the continuous predictor and the proportion (%) of settled larvae in three of the four settlement positions as response variables. Four univariate linear models were then fitted with the proportion of settled larvae in each settlement position as the response variable, and initial larval density as the continuous predictor. The appropriateness of all models was examined through visual inspection of residuals, and all figures were created using the ‘ggplot2’ package49 and ‘cowplot’ package50.
Source: Ecology - nature.com


